The Dual Impact of AI in Clinical Trials: Perspective
Journal of Engineering Research and Sciences, Volume 3, Issue 9, Page # 16-25, 2024; DOI: 10.55708/js0309002
Keywords: Artificial Intelligence, Clinical Trials, Patient Outcomes, Predictive Analytics, Healthcare Inequities
(This article belongs to the Special Issue on SP5 (Special Issue on Multidisciplinary Sciences and Advanced Technology 2024) and the Section Medical Informatics (MDI))
Export Citations
Cite
Yaqub, M. and He, L. (2024). The Dual Impact of AI in Clinical Trials: Perspective. Journal of Engineering Research and Sciences, 3(9), 16–25. https://doi.org/10.55708/js0309002
Muhammad Yaqub and Lan He. "The Dual Impact of AI in Clinical Trials: Perspective." Journal of Engineering Research and Sciences 3, no. 9 (September 2024): 16–25. https://doi.org/10.55708/js0309002
M. Yaqub and L. He, "The Dual Impact of AI in Clinical Trials: Perspective," Journal of Engineering Research and Sciences, vol. 3, no. 9, pp. 16–25, Sep. 2024, doi: 10.55708/js0309002.
The use of artificial intelligence in clinical trials opens the way for a period of transformation in which the potential to enhance efficiency, precision, and scope in clinical investigation is enormous. In this perspective, the promise and perils of AI regarding clinical trials are critically reviewed. On one hand, it's where AI can really make some revolutionary changes to the most critical aspects of trial design and execution—like patient recruitment, data management, and predictive analytics—which are now adaptive and more driven by data; on the other hand, AI applied in clinical trials also brings considerable problems related to data integrity, ethical considerations, and regulatory compliance. Such potential of AI to further biases, break patient confidences, and exacerbate already wide inequities in healthcare raises the need for vigilant oversight. As AI is rapidly evolving, so too are principles that guide transparency, fairness, and ethical rigors that guide their application in clinical trials. This work presents a case for strong AI frameworks that are subject to firm validation and ethical scrutiny, which will ensure that the benefits of AI are realized with reduced risks associated with it. If the field goes down this road, AI integration in clinical trials is going to be the real catalyst for innovation, which could translate into achieving better patient outcomes and expanding frontiers for medical science.
1. Introduction
One of the most extreme developments in medical research is the use of AI in clinical trials. Already available are emerging AI technologies offering enormous potential for efficiency, accuracy, and scalability in clinical trials.[1–3] . That promise is wrapped in a series of major challenges and key ethical concerns. There are many risks associated with implementing AI within clinical trials. Serious issues of data privacy, bias in algorithms, transparency, and the possible increase of inequities in health characterize a very difficult area of navigation. It also brings along several questions on the level of regulatory compliance, validity of the AI-driven outcomes, and whether healthcare is prepared to absorb full potential for AI while protecting the rights and well-being of the patient [4,5]. As summarized in Table 1, the literature highlights the transformative impact of AI on clinical trials, particularly in improving patient recruitment efficiency, enhancing data management, and addressing ethical and privacy concerns. These studies underscore both the potential benefits and the challenges that need to be navigated to fully integrate AI into clinical research.
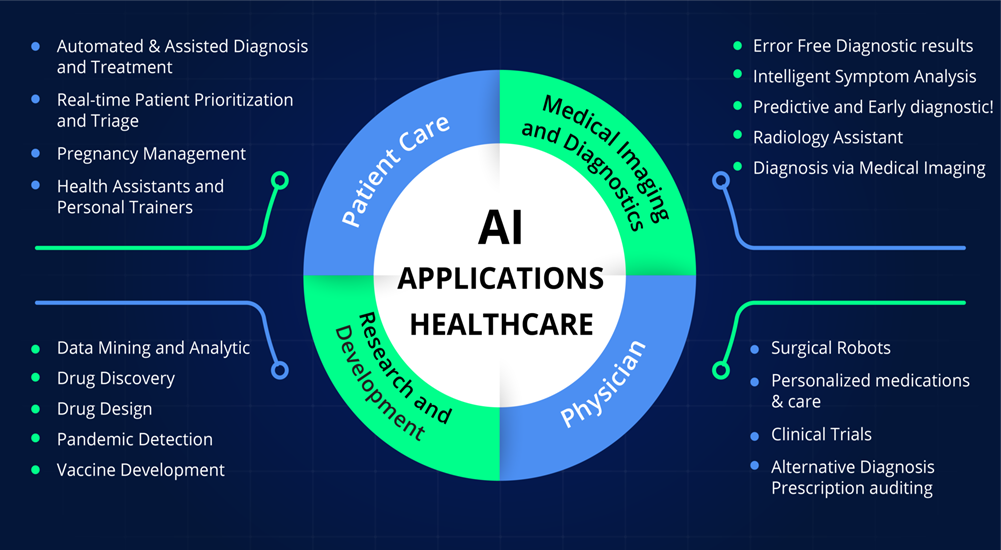
It is therefore advanced in this paper that the dual-edged potential of AI in the context of clinical trials demands an appropriate and integrative perspective of the optimistic possibilities and potential risks concomitant with it. Current advancements in this area, related ethical considerations, and regulatory challenges were looked at in order to provide a balanced view in respect of the optimization of clinical trials using AI and the critical issues that have to be tackled for its successful integration. It contributes to the discussion on responsible and effective application of AI about medical research, and subsequently toward an innovation-sound and ethical future for AI-aided clinical trials. Figure 1 illuminating the promising role of AI in healthcare.
Table 1: Key Literature on the Integration of Artificial Intelligence in Clinical Trials
Reference | Year | Title | Key Findings | Relevance to AI in Clinical Trials |
[6] | 2019 | High-performance medicine: the convergence of human and artificial intelligence | AI has the potential to significantly enhance the accuracy, efficiency, and personalization of healthcare, including in clinical trials. | Discusses the overall potential of AI in healthcare, highlighting its transformative impact on clinical trials. |
[7] | 2020 | The future of digital health with federated learning | Federated learning offers a way to leverage AI while preserving patient data privacy, a critical aspect of clinical trials. | Provides insights into maintaining data privacy in AI-driven clinical trials, a key ethical concern. |
[8] | 2019 | Optimizing patient recruitment with AI | AI can streamline patient recruitment by analyzing electronic health records to identify candidates more efficiently. | Focuses on how AI can address the significant challenge of patient recruitment in clinical trials. |
[9] | 2020 | International evaluation of an AI system for breast cancer screening | Demonstrates the accuracy of AI in medical imaging, with potential applications in clinical trial monitoring and outcome prediction | Illustrates the practical applications of AI in diagnostic processes relevant to clinical trials. |
[10] | 2021 | Overview of deep learning applications in medical image analysis | Reviews the state-of-the-art deep learning methods in medical image analysis, highlighting their potential in disease diagnosis and clinical trials. | Provides a comprehensive review of AI applications in medical imaging, relevant for trials involving imaging-based outcomes. |
[11] | 2020 | Ethical considerations in the deployment of AI in healthcare | Discusses the ethical implications of AI in healthcare, including issues of bias, transparency, and accountability. | Essential for understanding the ethical challenges AI presents in the clinical trial context. |
[12] | 2017 | Dermatologist-level classification of skin cancer with deep neural networks | AI achieved dermatology-level accuracy in skin cancer diagnosis, suggesting AI’s potential to improve diagnostic accuracy in trials. | Demonstrates the effectiveness of AI in diagnostics, relevant for trials focusing on dermatological conditions. |
[13] | 2017 | Artificial intelligence in healthcare: past, present, and future | Explores the development of AI in healthcare and its potential future applications, including clinical trials. | Provides historical context and future outlook on AI’s role in clinical trials. |
[14] | 2019 | The role of artificial intelligence in precision medicine | AI enables precision medicine approaches in clinical trials, allowing for more tailored treatment interventions. | Highlights how AI can enhance the personalization of clinical trials through precision medicine. |
[15] | 2018 | How artificial intelligence is changing drug discovery | AI accelerates the drug discovery process, including in | Discusses AI’s role in the early stages of clinical trials, particularly in drug discovery and testing. |
2. The Impact of AI in Medical Imaging
The effect of AI on clinical trials, even more so in the analysis of medical imaging, goes deep, as it helps in improving image analysis, smoothing trial workflows, and enhancing diagnostic precision. Speaking precisely, some of the ways AI can modify medical imaging in clinical trials include:
2.1. Automated Image Analysis and Interpretation
Medical imaging modalities, including but not limited to MRI, CT, and PET, are increasingly used in clinical trials for assessment of diseases process, treatment efficacy assessment, or biomarkers evaluation [16,17]. Traditional radiologist interpretation of images is very time-consuming and may be variable. Algorithms based on AI, especially those using deep learning techniques, can be programmed to look at medical imagery for patterns indicating lesions or other abnormalities that may well be too slight for the human eye to recognize. For example:
- AI-powered tools in an oncology trial can assist by automatic detection and quantification of tumor size, shape, and growth patterns in MRI or CT images, improving the assessment of treatment responses.
- AI in cardiology can analyze echocardiograms or cardiac MRIs to measure heart function, such as ejection fraction, wall motion, and other variables that provide insight into drug effects on cardiovascular health.
AI-driven automation decreases interpretation time for images, enhances consistency in the review of images, and decreases the probability of human-related errors. The outcome is speedier trial results and more valid results, as the imaging biomarkers could be studied more precisely.
2.2. Enhanced Image-Based Biomarker Discovery
Biomarkers are important in a clinical trial in monitoring disease process or treatment response [18]. For imaging, this could involve the detection of slight structural or functional alterations in tissues, which are indicative of the disease. Artificial Intelligence has this phenomenal capability to discover new imaging biomarkers by analyzing huge datasets, with subtle patterns that may evade human eyes [19]. For instance:
- So far, these models have been applied in neurology for brain MRI reviewing, considering a number of biomarkers for various diseases, such as Alzheimer’s disease and multiple sclerosis [20], which are grounded on different brain volume changes, cortical thickness, and white matter integrity.
- AI will find the radiomic features in oncology, such as microscopic variations either in tumor textures or shapes related to variations in patient outcomes, allowing early treatment efficacy predictions.
AI, therefore, applies to an improved ability in detection and quantification of complex biomarkers from imaging information, which enables better prediction of the progression of diseases and responses to pharmacological treatments. Consequently, this process has encouraged the formulation of more effective therapies and personalized ones during clinical trials, out of which comes the shortening of timelines for bringing efficacious therapies to market.
2.3. Real-Time Image Monitoring in Adaptive Trials
AI represents the backbone of adaptive clinical trials, most seen in real-time image monitoring during trials, for which imaging is an enabler of response assessment in patients and modifications to trials [21]. Some decisions regarding dosage, continuing or modifying treatment, or even cohort adjustments depend mostly on scheduled reviews. AI can give data-driven insights at the exact moment by analyzing real-time imaging results. For example, in oncology trials, the AI system can track day-to-day changes in tumor size, density, and vascularity from MRI or CT scans continuously, which, based on a poor response of a patient, clinicians can alter the regimen of treatment immediately. This will save precious time in making critical adjustments, therefore offering the best possible care for a patient. AI-driven image analysis also cuts down on human error; hence, allowing more consistent and objective assessments across large cohorts of patients. AI makes clinical trials more flexible, meaning that faster and more precise assessments will enable the creation of better treatments.
2.4. AI in Image-Based Inclusion/Exclusion Criteria
It often happens that medical imaging itself is crucial for the inclusion or exclusion of patients in clinical trials, depending on whether the extent of the disease, organic damage, or size of lesions meets the particular inclusion or exclusion criteria. Conventionally, radiologists would review such criteria manually by going through the imaging data; this is a very time-consuming process and may be prone to inaccuracies and inconsistencies. Artificial Intelligence solves this through automation of involved processes. It ensures the screening of the patients systematically and precisely based on the eligibility criteria brought about by the trial in mention [22].
For example, in neurological clinical trials, algorithms in AI can independently analyze MRI images to look for symptoms of brain atrophy or even lesions that will determine if a patient can be included or not. Similarly, AI in oncology research is able to evaluate the dimensions and dissemination of tumors, classifying patients into the stages of investigation accordingly. This automation simplifies the process of screening, enhances the accuracy in patient selection, and thereby accelerates participants’ recruitment, which has been a major bottleneck in many clinical trials.
2.5. Improved Patient Monitoring through Longitudinal Imaging
Longitudinal imaging [23] is very often necessary in many clinical trials, especially those that target chronic or progressive diseases; this usually requires the repeated scanning of patients over time to monitor changes in disease progression or therapeutic effects. AI enhances this process by analyzing sequential imaging data and pointing out subtle changes that may pass undetected by the human eye. For example, in neurodegenerative disease trials, AI can quantify the change in brain volume between time points to give early signs of disease progression or response to therapy, while in oncology trials, the rate of tumor shrinkage or detection of new metastases in follow-up scans from AI can continuously provide feedback on treatment efficacy. The potential to automatically analyze complex imaging data over long periods ensures increased precision in monitoring, allowing for adjustments in trials based on real patient responses and therefore enabling rapid and true assessments of the efficacy of drugs.
2.6. Reducing Variability in Imaging Interpretations
One major challenge facing clinical trials involving imaging modalities is the variation in the interpretation of the same image by different radiologists. Such variability leads to heterogenous data that might affect the outcome of such research. Artificial intelligence applications overcome this problem by applying uniform criteria to all image analyses, hence enabling uniform interpretation of images irrespective of the trial site or the personnel involved.
While artificial intelligence systems can, for example, measure such characteristics of lesions objectively for conditions like multiple sclerosis or cancers where the quantity and dimensions of lesions are a primary critical endpoint in clinical trials on all subjects. This permits a reduction of inter-reader variability and thus provides an assessment platform that is both standardized and reliable. The consistency of image reading is particularly essential in multisite clinical trials, where the results of image reading coming from different sites should be comparable. By mitigating the variability of humans, artificial intelligence enhances the robustness and reliability of trial data, leading to a more exact conclusion.
2.7. AI for Enhanced Image-Guided Drug Delivery
The use of imaging in delivering drugs to a specific part of the body during most clinical targeted therapies, especially in cancer treatment, is critical. Artificial intelligence enhances the precision in such analyses of image data and hence could allow therapeutic agents to reach the sites with greater accuracy. The examples are that AI, in radiotherapy research, can analyze CT or MRI scans to delineate tumor margins with high precision so that radiation is delivered onto cancerous tissues while sparing other healthy ones. For example, in trials with nanoparticle or antibody-drug conjugates, AI can aid in determining optimum delivery sites and/or real-time drug dispersion with companion imaging techniques like PET or SPECT. Greater precision reduces the risk of unwanted off-target effects, improves the overall safety profile of these therapeutic interventions, and enhances the possibility of successful realization of the intended outcomes of the therapeutic interventions themselves. While AI-driven imaging guidance in drug delivery serves the dual purpose of enhancing effectiveness, the most important part is that it optimizes trial protocols by enhancing efficiency and productivity within clinical research [24].
3. Case Studies
The following are some case studies that give an idea of the realistic implementation of AI in clinical trials. It underlines and identifies real practical examples in order to explain exactly how AI impacted different stages of clinical research.
3.1. IBM Watson for Drug Discovery – Accelerating Cancer Research
IBM Watson for Drug Discovery has been employed by various pharmaceutical companies and research institutions to accelerate the identification of potential therapeutic targets and biomarkers for diseases like cancer. Working with the Barrow Neurological Institute, Watson [25] uncovered five new ALS-related gene candidates in less than two months’ work that, if done by human researchers, would have taken years. Scanning through thousands of scientific papers and clinical trial records, AI was able to quickly create links between genes and ALS, accelerating the pathway to finding new medications.
The AI-driven literature and data mining of Watson reduced the time it took to sift through datasets that were too complex; this hastened the early phases of clinical trials and hypothesis generation. This case underlines the capability of AI in manipulating large volumes of unstructured data, hence reinforcing trial design and biomarker identification, especially in complex diseases like ALS and cancer.
3.2. Tempus – Personalized Oncology Trials
Tempus is a Chicago-based technology company that uses AI to study clinical and molecular data to personalize cancer treatment. Its platform collates clinical data, including the genetic profile of patients, in order to match them with suitable clinical trials.
Tempus [26] has partnered with the University of Chicago Medicine to incorporate its AI platform into clinical trial matching at the hospital. In return, it analyzes the genomic sequencing data and clinical records to identify appropriate clinical trials for cancer patients and help match the right patients into the relevant oncology trials. This reduced the time taken by the system in matching patients with trials, while the trials the patients were being subjected to were more accurate. AI-driven patient matching works to improve enrollment rates, normally bottlenecks to oncology research. Additionally, this will enable the improvement of prospects for therapeutic success on account of the closer genetic profile alignment of a patient with trial parameters for more tailored and effective treatments.
3.3. Medidata – AI for Virtual Clinical Trial
Medidata is a company focused on cloud-based solutions for life sciences, having come up with an AI platform that allows the implementation of virtual clinical trials. The approach thereby minimizes physical visits and allows for remote data collection, which applies to rare diseases or pandemics, such as COVID-19. Medidata [27] conducted virtual trials of a candidate COVID-19 vaccine by implementing AI for remote monitoring of patient data. The AI-enabled platform collected data from wearables and patient-reported outcomes, while making the processing easier with a reduced administrative burden. This was interpreted as monitoring patients in real-time, without asking patients to travel to trial sites.
Virtual trials by Medidata removed geographical boundaries and allowed more patients to participate in parts of the world where access to clinical trial sites was at a premium. The integration of data from wearables enabled by AI also improved monitoring of patient health metrics, thus allowing quicker safety assessments and flexibility in the management of trials. That has been particularly useful during the pandemic for running trials in a decentralized manner and finds wider application now.
3.4. AI in Adaptive Clinical Trials – Novartis and Microsoft
Novartis has allied with Microsoft to use AI in the design of adaptive clinical trials where real-time changes can be made to trial protocols based on intermediate outcomes [28]. This becomes particularly important in oncology, where patient responses can be highly variable. AI models, developed under the partnership between Novartis and Microsoft, predicted optimal dosing levels and signals of early treatment efficacy. For instance, AI models spotted subgroups of more responsive patients during a recent breast cancer trial, allowing for adaptive changes in the protocol of this trial focused on these subgroups.
Adaptation in trials-in fact, making changes according to responses-allows for efficiency in research and safety for the patient. AI enhances that ability to dynamically alter trial parameters, reducing time and cost it takes in new treatments. For example, in the case of this AI-driven trial on breast cancer, the number of patients exposed to ineffective treatments was reduced while hastening the approval process of successful therapies.
3.5. Benevolent AI – Accelerating Drug Repurposing
Benevolent AI applies the power of AI to find new drug candidates and indications that find new uses for already-approved drugs [29]. It reads through biomedical data like clinical trials, patents, and scientific literature to identify new potential uses for already-approved drugs. In the COVID-19 pandemic, Benevolent AI mentioned the use of baricitinib-a rheumatoid arthritis medication-as a likely candidate against COVID-19. The AI system curbed through mountains of data and suggested that the drug could reduce the cytokine storm that happens in serious cases of COVID-19, and hence it is an ideal candidate for repurposing.
Clinical trials started for baricitinib, and it proved very beneficial for COVID-19 patients. The drug was able to garner use authorization in emergency situations from the FDA. This is just one example in which AI accelerates a process that, without AI, involves tremendous time and resources: finding new uses for existing drugs. AI analyzes cross-domain datasets, driving much higher efficiency in drug repurposing.
3.6. Verge Genomics – AI for ALS Clinical Trials
Verge Genomics employs AI and machine learning to map the human genome for new treatments of neurodegenerative diseases such as ALS [30]. In collaboration with the Cedars-Sinai Medical Center, Verge has fielded its AI platform against ALS patient data and surfaced novel targets for potentially informed clinical trials. In this respect, by applying AI to identify genetic patterns, Verge can accelerate the preclinical stages and move promising candidates into clinical trials more rapidly than would have otherwise been possible. The AI platform at Verge automated target identification and shaved huge margins off ALS drug development times and costs. The rapid pace from identification to clinical trials punts on AI’s power for accelerating the initial parts of neurodegenerative disease clinical research, where advances usually take so much time.
These case studies epitomize how AI can transform everything from patient recruitment and data analysis to adaptive trial designs and drug repurposing within the clinical trial space. AI-driven tools enhance clinical research speed, efficiency, and precision-enabling better outcomes in oncology, neurodegenerative diseases, and pandemic response efforts. These examples demonstrate great strides that have been taken in real-world uses where AI makes the clinical trial more efficient, personalized, and accessible.
4. The Promise of AI in Clinical Trials
Clinical trials powered by AI can bring about a wealth of advantages to almost flip a new leaf in medical research and treatment. This starts from the patient selection stage and takes the shapes of the analysis, monitoring, and outcome prediction for clinical trials. document.
Table 2: Summary of the Promises and Perils of AI in Clinical Trials
Aspect | Promises | Perils |
Efficiency [31] | Streamlines data analysis and patient selection. | – Over-reliance on AI could lead to overlooking nuanced clinical insights. |
Accuracy [32] | – AI models can process large large datasets, identifying patterns beyond human capability. | – Potential for bias in AI algorithms due to biased training data. |
Cost-Effectiveness [33] | – Reduces costs by automating routine tasks and improving trial design. | – High initial costs for AI implementation and integration. |
Patient Stratification [25] | – Identifies optimal patient subgroups for targeted therapies. | – Risk of excluding underrepresented groups, leading to inequitable outcomes. |
Speed [34] | – Accelerates the timeline of trials by predicting outcomes and optimizing processes. | – Speed at the expense of thoroughness may compromise the trial’s integrity. |
Regulatory Compliance [35] | – Assists in meeting regulatory requirements by maintaining accurate records and analysis. | – Regulatory bodies may struggle to keep pace with rapidly evolving AI technologies. |
Ethical Considerations [36] | – AI can help ensure ethical trial designs by identifying potential issues in advance. | – Ethical dilemmas around data privacy, consent, and the use of AI in decision-making. |
Interpretability [37] | – Enhances understanding of complex data through advanced analytics and visualization tools. | – Black-box nature of some AI models may hinder transparency and trust in AI-driven decisions. |
4.1. Enhanced Patient Recruitment and Retention
Patient recruitment and retention are the biggest challenges to clinical trials, namely, enrolling eligible patients or participants. The traditional model of doing things relies on manual efforts, which become time-consuming, prone to mistakes, and cumbersome. AI can fast-track patient recruitment by analyzing voluminous electronic health records and other medical data for suitable candidates with greater speed and accuracy. AI Algorithms can thus be trained to provide an estimate of the patient retention probability by detecting those factors that could influence a participant’s likelihood of completing a trial, thus improving retention rates and overall success of a trial.
4.2. Improve how one manages and analyzes data
Clinical trials involve huge volumes of data, from patient demographics to clinical measurements, genomic data, and image data. Management and analysis operations for this kind of data are bulky and resource intensive. AI has the potential to innovate data management through automating means of processing and analyzing big datasets to researchers for them to find out hidden patterns and insights that, otherwise, would not be evident in the use of conventional statistical methods. Through machine learning algorithms, one is able to come up with the correlations, predict the outcome, and even hypothesize new hypotheses that enable researchers to fast track the processes in research activities, thereby increasing the accuracy of results realized in trials.
4.3. Personalized Medicine and Adaptive Trials
AI also deepens analysis of datasets in ways that foster the development of personalized medicine approaches within clinical trials. In this regard, AI would be useful in developing treatment trials through integrating genomic, phenotypic, and clinical data in tailoring treatments to the characteristics of the individual patient. This customization will maximize the possibilities of success of the treatment while minimizing its side effects. Moreover, AI can support adaptive designs whereby the parameters of the trial are adapted based on the results of the interim analysis. A very key implication of adaptability is running quite a lot more efficient trials whereby treatments that promise much earlier are fast-tracked and less effective de-prioritized.
4.4. Real-time monitoring and predictive analytics
Clinical trials monitoring may leverage the power of AI since such real-time analysis of patient data will be useful in quickly establishing safety problems and adverse events. Besides, predictive analytics, powered by AI, will project the outcome from early patient data so that one can decide wisely. This ability is most important for the early identification of possible risks and benefits during a trial process for more effective and safer treatments.
5. The Perils of AI in Clinical Trials
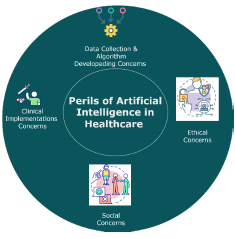
Although the potential benefits of AI in clinical trials are huge, so are the challenges to be addressed for its ethical and effective application in this context. Figure 2 presents the major perils of AI clinical trials.
5.1. Data Privacy and Security Concern
The application of AI in clinical trials presupposes the collection and analysis of large amount of personal health data. This gives more rise to concerns of privacy and data security, particularly, of sensitive medical information. Ensuring AI systems are complaint with data protection regulation, such as General Data Protection Regulation in Europe is critically important. There is also the risk that AI systems might be vulnerable to cyberattacks, which could lead to a breach in patient confidentiality and the data being misused.
5.2. Algorithmic Bias and Fairness
AI algorithms are only as good as the data on which they have been trained. If the training data is biased or unrepresentative, it can mean that AI systems produce biased outcomes, affecting differentially certain groups of patients. This will become of major concern in clinical trials, where biased algorithms could permit unequal access to treatment or inaccurate predictions about treatment efficacy for different demographic groups. Ensuring AI systems are trained on diverse and representative datasets is critical to mitigating these risks.
5.3. Transparency and Explainability
Such concerns also exist with regard to the fact that most AI systems, especially those involving deep learning, are often “black boxes” for which it can be pretty difficult to understand how they came up with certain decisions or predictions. This may then cause problems during clinical trials, where maximum effort is made to understand the logic behind treatment decisions and the subsequent results as clearly as possible. In that light, it is incumbent on researchers and regulators to commence the process of developing AI systems that are not only accurate but also explainable, so that use in clinical trials is transparent and hence trustable.
5.4. Algorithm
The infusion of AI into clinical trials will obviously raise a new dimension of regulatory challenges since prevailing frameworks may not be fully ready to face the challenge of the complexity of AI-driven research. This will require up-to-date guidelines to be developed, taking into account the specificity connected with the AI applications, such as algorithm validation, data governance, and ethical concerns. As shown in Table 2, the integration of AI in clinical trials presents a range of promises, including enhanced precision and efficiency, alongside significant perils, such as ethical dilemmas and data privacy concerns, highlighting the need for careful consideration in its application. Also, a series of ethical questions arise from the use of AI in clinical trials about how automation might eventually substitute human judgment in areas critical to patient care and research. The challenge would be to balance the benefits of AI with the needs for human oversight and ethical accountability.
6. Recommendations for Future Research
- Future research emphasis should be to enhance the interpretability and transparency of complex models, such as those dealing with deep learning, toward clinicians and regulators to instill trust in AI-driven decisions made during clinical trials.
- Assess how AI integrates different types of data-imaging, genomics, clinical information-into one analysis that has the potential to advance personalized treatment strategies, and subsequently overall trial outcomes, for each data type.
- Strive for models applying real-world evidence through artificial intelligence and wearables to EHRs and patient-reported outcomes to develop better generalizability and applicability of findings from clinical trials.
- Design a framework directed towards the elimination of bias in artificial intelligence models in order to achieve equality regarding age, gender, and ethnicity. Further research works shall, therefore, be channeled towards the improvement of training datasets to make the AI assessment representative and inclusive.
- The development of the AI framework should focus on international legislation requirements. Research is to be directed to make AI systems used in trials transparent and auditable, which again justifies that privacy and safety regulations from organizations such as the FDA and EMA are met.
7. Conclusion
Artificial intelligence might be very instrumental in changing clinical trials into medical research with the potential advantages of efficiency, accuracy, and scalability. Such benefits are hedged with major challenges that need to be navigated with care for ensuring ethical and effective applications of AI. It is in the research community’s best interest to address issues of data privacy, algorithm bias, transparency, and regulation to realize the power of AI in moving clinical trials forward without putting a patient’s rights and well-being in jeopardy. In other words, AI in clinical research has a future if it is able to strike proper balance between innovation and ethical responsibility in ensuring that this technology gets used to its fullest for the betterment of all.
Conflict of Interest
The authors declare no conflict of interest.
- Askin, D. Burkhalter, G. Calado, S. El Dakrouni, Artificial Intelligence Applied to clinical trials: opportunities and challenges, Health and Technology, vol. 13, no. 2, 2023, doi:10.1007/s12553-023-00738-2.
- S. Piantadosi, Clinical trials: a methodologic perspective, John Wiley & Sons, 2024.
- H. Saeed, I. El Naqa, Artificial intelligence in clinical trials, Springer: 453–501, 2022.
- B.C. Stahl, Artificial Intelligence for a Better Future An Ecosystem Perspective on the Ethics of AI and Emerging Digital Technologies, 2021.
- W.H. Organization, Ethics and governance of artificial intelligence for health: large multi-modal models. WHO guidance, World Health Organization, 2024.
- E.J. Topol, High-performance medicine: the convergence of human and artificial intelligence, Nature Medicine, vol. 25, no. 1, 2019, doi:10.1038/s41591-018-0300-7.
- N. Rieke, J. Hancox, W. Li, F. Milletarì, H.R. Roth, S. Albarqouni, S. Bakas, M.N. Galtier, B.A. Landman, K. Maier-Hein, S. Ourselin, M. Sheller, R.M. Summers, A. Trask, D. Xu, M. Baust, M.J. Cardoso, “The future of digital health with federated learning,” Npj Digital Medicine, vol. 3, no. 1, 2020, doi:10.1038/s41746-020-00323-1.
- M. Woo, “Trial by artificial intelligence,” Nature, vol. 573, no. 7775, pp. S100–S102, 2019.
- S.M. McKinney, M. Sieniek, V. Godbole, J. Godwin, N. Antropova, H. Ashrafian, T. Back, M. Chesus, G.C. Corrado, A. Darzi, M. Etemadi, F. Garcia-Vicente, F.J. Gilbert, M. Halling-Brown, D. Hassabis, S. Jansen, A. Karthikesalingam, C.J. Kelly, D. King, J.R. Ledsam, D. Melnick, H. Mostofi, L. Peng, J.J. Reicher, B. Romera-Paredes, R. Sidebottom, M. Suleyman, D. Tse, K.C. Young, et al., “International evaluation of an AI system for breast cancer screening,” Nature, vol. 577, no. 7788, 2020, doi:10.1038/s41586-019-1799-6.
- T. Dhar, N. Dey, S. Borra, R.S. Sherratt, “Challenges of Deep Learning in Medical Image Analysis—Improving Explainability and Trust,” IEEE Transactions on Technology and Society, vol. 4, no. 1, 2023, doi:10.1109/tts.2023.3234203.
- S. Gerke, T. Minssen, G. Cohen, Ethical and legal challenges of artificial intelligence-driven healthcare, 2020, doi:10.1016/B978-0-12-818438-7.00012-5.
- A. Esteva, B. Kuprel, R.A. Novoa, J. Ko, S.M. Swetter, H.M. Blau, S. Thrun, “Dermatologist-level classification of skin cancer with deep neural networks,” Nature, vol. 542, no. 7639, 2017, doi:10.1038/nature21056.
- F. Jiang, Y. Jiang, H. Zhi, Y. Dong, H. Li, S. Ma, Y. Wang, Q. Dong, H. Shen, Y. Wang, Artificial intelligence in healthcare: Past, present and future, Stroke and Vascular Neurology, vol. 2, no. 4, 2017, doi:10.1136/svn-2017-000101.
- T.M. Maddox, Clinical Decision Support in Statin Prescription – What We Can Learn from a Negative Outcome, JAMA Cardiology, vol. 6, no. 1, 2021, doi:10.1001/jamacardio.2020.4756.
- N. Fleming, “How artificial intelligence is changing drug discovery,” Nature, vol. 557, no. 7707, 2018, doi:10.1038/d41586-018-05267-x.
- F.Y. Chiu, Y. Yen, Imaging biomarkers for clinical applications in neuro-oncology: current status and future perspectives, Biomarker Research, vol. 11, no. 1, 2023, doi:10.1186/s40364-023-00476-7.
- M. Yaqub, F. Jinchao, M.S. Zia, K. Arshid, K. Jia, Z.U. Rehman, A. Mehmood, “State-of-the-art CNN optimizer for brain tumor segmentation in magnetic resonance images,” Brain Sciences, vol. 10, no. 7, 2020, doi:10.3390/brainsci10070427.
- K.D. Davis, N. Aghaeepour, A.H. Ahn, M.S. Angst, D. Borsook, A. Brenton, M.E. Burczynski, C. Crean, R. Edwards, B. Gaudilliere, G.W. Hergenroeder, M.J. Iadarola, S. Iyengar, Y. Jiang, J.T. Kong, S. Mackey, C.Y. Saab, C.N. Sang, J. Scholz, M. Segerdahl, I. Tracey, C. Veasley, J. Wang, T.D. Wager, A.D. Wasan, M.A. Pelleymounter, “Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: challenges and opportunities,” Nature Reviews Neurology, vol. 16, no. 7, 2020, doi:10.1038/s41582-020-0362-2.
- A. Prelaj, V. Miskovic, M. Zanitti, F. Trovo, C. Genova, G. Viscardi, S.E. Rebuzzi, L. Mazzeo, L. Provenzano, S. Kosta, M. Favali, A. Spagnoletti, L. Castelo-Branco, J. Dolezal, A.T. Pearson, G. Lo Russo, C. Proto, M. Ganzinelli, C. Giani, E. Ambrosini, S. Turajlic, L. Au, M. Koopman, S. Delaloge, J.N. Kather, F. de Braud, M.C. Garassino, G. Pentheroudakis, C. Spencer, et al., Artificial intelligence for predictive biomarker discovery in immuno-oncology: a systematic review, Annals of Oncology, vol. 35, no. 1, 2024, doi:10.1016/j.annonc.2023.10.125.
- B. Rossi, B. Santos-Lima, E. Terrabuio, E. Zenaro, G. Constantin, Common Peripheral Immunity Mechanisms in Multiple Sclerosis and Alzheimer’s Disease, Frontiers in Immunology, vol. 12, 2021, doi:10.3389/fimmu.2021.639369.
- E.T. Hébert, C.K. Ra, A.C. Alexander, A. Helt, R. Moisiuc, D.E. Kendzor, D.J. Vidrine, R.K. Funk-Lawler, M.S. Businelle, “A mobile just-in-time adaptive intervention for smoking cessation: Pilot randomized controlled trial,” Journal of Medical Internet Research, vol. 22, no. 3, 2020, doi:10.2196/16907.
- S. Prabhu, K. Prasad, A. Robels-Kelly, X. Lu, AI-based carcinoma detection and classification using histopathological images: A systematic review, Computers in Biology and Medicine, vol. 142, 2022, doi:10.1016/j.compbiomed.2022.105209.
- W.M. van Oostveen, E.C.M. de Lange, “Imaging techniques in Alzheimer’s disease: a review of applications in early diagnosis and longitudinal monitoring,” International Journal of Molecular Sciences, vol. 22, no. 4, 2110, 2021.
- W. He, Z. Zhang, Y. Luo, R.T.K. Kwok, Z. Zhao, B.Z. Tang, Recent advances of aggregation-induced emission materials for fluorescence image-guided surgery, Biomaterials, vol. 288, 2022, doi:10.1016/j.biomaterials.2022.121709.
- A. Smart, P. Martin, M. Parker, Tailored medicine: Whom will it fit? The ethics of patient and disease stratification, Bioethics, vol. 18, no. 4, 2004, doi:10.1111/j.1467-8519.2004.00400.x.
- Z. Chaudhary, C. Lungren, A. Shah, “Tempus: Reshaping the Landscape of Cancer Care with Revolutionary Artificial Intelligence,” Teens in Health AI in Healthcare Summer 2023 Journal Teens in Health Is a Teen Led Organization That Aims to Provide Open Access to Biological Research Skill Development through Researching and Writing Articles, 148, 2023.
- K.H. Zou, C. Vigna, A. Talwai, R. Jain, A. Galaznik, M.L. Berger, J.Z. Li, The Next Horizon of Drug Development: External Control Arms and Innovative Tools to Enrich Clinical Trial Data, Therapeutic Innovation and Regulatory Science, vol. 58, no. 3, 2024, doi:10.1007/s43441-024-00627-4.
- D. Hartl, V. de Luca, A. Kostikova, J. Laramie, S. Kennedy, E. Ferrero, R. Siegel, M. Fink, S. Ahmed, J. Millholland, A. Schuhmacher, M. Hinder, L. Piali, A. Roth, Translational precision medicine: an industry perspective, Journal of Translational Medicine, vol. 19, no. 1, 2021, doi:10.1186/s12967-021-02910-6.
- M. Shanmuga Sundari, H.R. Penthala, A. Mogullapalli, M.M. Ammangatambu, “AI-Based Personalized Drug Treatment,” Artificial Intelligence and Machine Learning in Drug Design and Development, 369–406, 2024.
- J. Geraci, R. Bhargava, B. Qorri, P. Leonchyk, D. Cook, M. Cook, F. Sie, L. Pani, “Machine learning hypothesis-generation for patient stratification and target discovery in rare disease: our experience with Open Science in ALS,” Frontiers in Computational Neuroscience, vol. 17, 2023, doi:10.3389/fncom.2023.1199736.
- S.M. Williamson, V. Prybutok, Balancing Privacy and Progress: A Review of Privacy Challenges, Systemic Oversight, and Patient Perceptions in AI-Driven Healthcare, Applied Sciences (Switzerland), vol. 14, no. 2, 2024, doi:10.3390/app14020675.
- R. Schwartz, R. Schwartz, A. Vassilev, K. Greene, L. Perine, A. Burt, P. Hall, Towards a standard for identifying and managing bias in artificial intelligence, US Department of Commerce, National Institute of Standards and Technology, 2022.
- N. Hendrix, D.L. Veenstra, M. Cheng, N.C. Anderson, S. Verguet, “Assessing the Economic Value of Clinical Artificial Intelligence: Challenges and Opportunities,” Value in Health, vol. 25, no. 3, 2022, doi:10.1016/j.jval.2021.08.015.
- A. Jungbauer, G. Ferreira, M. Butler, S. D’Costa, K. Brower, A. Rayat, R. Willson, “Status and future developments for downstream processing of biological products: Perspectives from the Recovery XIX yield roundtable discussions,” Biotechnology and Bioengineering, 2024.
- P.G.R. de Almeida, C.D. dos Santos, J.S. Farias, “Artificial Intelligence Regulation: a framework for governance,” Ethics and Information Technology, vol. 23, no. 3, 2021, doi:10.1007/s10676-021-09593-z.
- A. Nassar, M. Kamal, “Ethical Dilemmas in AI-Powered Decision-Making: A Deep Dive into Big Data-Driven Ethical Considerations,” International Journal of Responsible Artificial Intelligence, vol. 11, no. 8, 2021.
- E. Nasarian, R. Alizadehsani, U.R. Acharya, K.-L. Tsui, “Designing interpretable ML system to enhance trust in healthcare: A systematic review to proposed responsible clinician-AI-collaboration framework,” Information Fusion, 102412, 2024.
- Muhammad Yaqub, Lan He, “Energy-Optimized Smart Transformers for Renewable-Rich Grids”, Journal of Engineering Research and Sciences, vol. 4, no. 10, pp. 21–28, 2025. doi: 10.55708/js0410003
- Muhammad Yaqub, Lan He, “AI-Powered Decision Support in SAP: Elevating Purchase Order Approvals for Optimized Life Sciences Supply Chain Performance”, Journal of Engineering Research and Sciences, vol. 4, no. 8, pp. 41–49, 2025. doi: 10.55708/js0408005
- Muhammad Yaqub, Lan He, “Magnetic AI Explainability: Retrofit Agents for Post-Hoc Transparency in Deployed Machine-Learning Systems”, Journal of Engineering Research and Sciences, vol. 4, no. 8, pp. 31–40, 2025. doi: 10.55708/js0408004
- Muhammad Yaqub, Lan He, “Comparative Analysis of Supervised Machine Learning Models for PCOS Prediction Using Clinical Data”, Journal of Engineering Research and Sciences, vol. 4, no. 6, pp. 16–26, 2025. doi: 10.55708/js0406003
- Muhammad Yaqub, Lan He, “Fire Type Classification in the USA Using Supervised Machine Learning Techniques”, Journal of Engineering Research and Sciences, vol. 4, no. 6, pp. 1–8, 2025. doi: 10.55708/js0406001
