A Swarm-Based Clinical Validation Framework of Artificial Intelligence Solutions for Non-Communicable Diseases
Journal of Engineering Research and Sciences, Volume 2, Issue 9, Page # 1-11, 2023; DOI: 10.55708/js0209001
Keywords: Swarm Intelligence, Clinical Validation, Framework, Non-Communicable Diseases, Diagnostic Accuracy, Personalized Treatment
(This article belongs to the Special Issue on SP3 (Special Issue on Multidisciplinary Sciences and Advanced Technology 2023) and the Section Medical Informatics (MDI))
Export Citations
Cite
Kioskli, K. , Papastergiou, S. and Fotis, T. (2023). A Swarm-Based Clinical Validation Framework of Artificial Intelligence Solutions for Non-Communicable Diseases. Journal of Engineering Research and Sciences, 2(9), 1–11. https://doi.org/10.55708/js0209001
Kitty Kioskli, Spyridon Papastergiou and Theofanis Fotis. "A Swarm-Based Clinical Validation Framework of Artificial Intelligence Solutions for Non-Communicable Diseases." Journal of Engineering Research and Sciences 2, no. 9 (September 2023): 1–11. https://doi.org/10.55708/js0209001
K. Kioskli, S. Papastergiou and T. Fotis, "A Swarm-Based Clinical Validation Framework of Artificial Intelligence Solutions for Non-Communicable Diseases," Journal of Engineering Research and Sciences, vol. 2, no. 9, pp. 1–11, Sep. 2023, doi: 10.55708/js0209001.
Non-communicable diseases (NCDs) present complex challenges in patient care. Artificial Intelligence (AI) offers transformative potential, but its implementation requires addressing key issues. This study proposes a swarm intelligence-inspired clinical validation framework for NCDs, promoting openness, trustworthiness, and continuous self-validation. The framework creates a collaborative environment, connecting healthcare entities, patients, caregivers, and professionals. The swarm-based approach enhances diagnostic accuracy, enables personalized treatment, improves prognosis, supports clinical decision-making, engages patients, enables real-time monitoring, and promotes continuous learning. These implications have the power to revolutionize NCD management and improve patient outcomes.
1. Introduction
Non-communicable diseases (NCDs) pose a significant challenge in patient care due to their complex nature. NCDs encompass a diverse range of chronic conditions, including cardiovascular diseases, cancer, diabetes, and respiratory disorders, among others. These diseases are typically long-lasting and progress slowly, requiring comprehensive and multidisciplinary management strategies [1]. The complexity of patient care arises from several factors, such as the interplay of genetic, environmental, and lifestyle factors in disease development and progression [2]. As a result, NCDs often go undetected until they reach an advanced stage, leading to poorer outcomes [3]. Moreover, NCDs often involve multiple organ systems and coexisting comorbidities, necessitating a holistic approach to treatment and care [1]. This complexity poses big challenge for health professionals on developing and applying a more personalised care, optimised treatment, and adverse event prediction that only an advanced computational ability could address [4,5]. Additionally, the management of NCDs relies not only on medical interventions but also on behavioral modifications, including lifestyle changes and adherence to long-term treatment plans [2]. NCDs have a substantial impact on global health, affecting a large number of people and causing a significant number of deaths. According to the WHO, NCDs are responsible for approximately 41 million deaths each year, which accounts for 71% of all global deaths. These diseases affect individuals across all regions of the world, regardless of their income level, and their prevalence is continuously increasing. It is estimated that NCDs contribute to 15 million premature deaths in people between the ages of 30 and 69 years [1].
Artificial Intelligence (AI) has emerged as a promising technology with the potential to revolutionize healthcare by enhancing diagnosis, treatment, and overall patient care. AI algorithms can process vast amounts of healthcare data, including electronic health records, medical imaging, genomic data, and scientific literature, to generate valuable insights and support clinical decision-making [6]. Machine learning algorithms can identify patterns and correlations in data, leading to improved accuracy in disease diagnosis and prognosis prediction [7]. Additionally, AI-based systems can assist healthcare providers in tailoring treatment plans through personalized medicine approaches, considering individual patient characteristics, genetic profiles, and responses to therapies [8] Moreover, AI applications such as natural language processing and chatbots can enhance patient engagement, facilitate remote monitoring, and provide timely health information and support [9]. However, the integration of AI into healthcare systems requires careful attention to ethical considerations, privacy and security safeguards, and effective collaboration between healthcare professionals and AI developers [8]. Despite these challenges, the potential of AI to revolutionize healthcare by improving efficiency, accuracy, and patient outcomes is a compelling area of research and development.
The healthcare sector operates under stringent regulations to ensure patient safety and efficacy of interventions, including those involving AI. Before an AI innovation can be incorporated into standard clinical practice, it must undergo clinical validation and obtain regulatory approval [10]. One of the challenges in developing widely applicable AI solutions is the limited availability of diverse training data, often sourced from a small number of institutions or hospitals, leading to potential issues with cross-population generalizability [11]. To address this, best practices recommend conducting both internal validation, where the AI model’s performance is assessed using a separate set of data points from the same source used for training, and external validation, which evaluates the general applicability of the model using data from different healthcare sites [12].
Clinical validation is a multifaceted process that goes beyond the evaluation of AI models on internal and external data sources. It aims to address the complexities of real-world clinical practice and encompasses considerations of clinical utility, workflow integration, and stakeholder responses [12]. AI performance alone does not guarantee improved clinical outcomes; it must be evaluated in the context of its clinical usefulness [8]. Factors such as AI explainability, ethical considerations, patient privacy, and legal implications should also be considered [11]. Adopting a multidisciplinary approach that involves all stakeholders from the early stages is crucial for the successful implementation of AI systems in healthcare practice [11]. Collaboration among AI researchers, healthcare providers, regulators, and patients is necessary to ensure that AI technologies align with clinical needs, are ethically sound, and integrate seamlessly into existing workflows [8].
In line with our vision, we aim to tackle the challenges associated with the widespread implementation of AI by proposing a swarm intelligence inspired clinical validation framework, for NCDs, which would be open and trustworthy and incorporate advanced continuous self-validation capabilities. The proposed framework leverages a swarm intelligence approach to establish a self-organized intelligence environment, bringing together individual healthcare (HC) entities, patients, caregivers, and healthcare professionals (HCPs) to foster collaborative interactions within the healthcare ecosystem. Inspiration will be drawn from biological swarm formations, enriching the framework’s design and functionality.
This paper is structured as follows: Section 2 describes our objectives. Section 3 provides a review of the relevant literature and highlights how this research advances beyond the current state of the art. Furthermore, Section 4 presents the adopted methodology in detail. Section 5 explores the swarm intelligence network establishment and its capabilities, while Section 6 delves into the evaluation methodology. Sections 7 and 8 discuss the practical and clinical implications of our work. Finally, the paper concludes with the main findings and suggestions for future work in Section 9. The novelty of this study lies in the proposal of a swarm intelligence-inspired clinical validation framework for NCDs, which fosters collaborative interactions among healthcare entities, patients, caregivers, and healthcare professionals, drawing inspiration from biological swarm formations. This research advances beyond the current state of the art by integrating advanced continuous self-validation capabilities and creating an open and trustworthy intelligence environment within the healthcare ecosystem.
2. Objectives
This swarm-based framework aims to enhance the process of clinical validation and consequently, clinical practice. It addresses the challenges posed by data biases and the limited generalizability of models caused by the prevailing research practice of relying on data from a single site for training and attempting to apply the resulting model to various sites. Additionally, it promotes the utilization of electronic Patient-Reported Outcome Measures (PROMs) and Patient-Reported Experience Measures (PREMs), as well as the continuous monitoring of patients. Furthermore, it emphasizes the incorporation of human factors into all aspects of the proposed technological intervention.
The framework has defined six ambitious interdisciplinary objectives that adhere to the SMART criteria (i.e., Specific, Measurable, Attainable, Realistic, and Time-bound). These objectives are outlined below:
- Utilize Swarm Intelligence (SI) capabilities to develop an innovative health-community-driven approach for clinical validation, aimed at improving personalized interventions, quality of life, and well-being for patients with NCDs.
- Enhance the accuracy, management, and effective coordination of healthcare professionals in clinical validation to advance personalized early risk prediction, prevention, and intervention strategies for patients.
- Streamline and enhance the validation process for AI solutions, incorporating swarm learning techniques and continuous learning methodologies.
- Ensure the privacy and security of data, as well as the AI and machine learning systems employed, in compliance with relevant regulations.
- Establish standard operating procedures for integrating AI into healthcare, specifically in supporting clinical decision-making for treatment and care.
- Measure the cost-effectiveness of AI-assisted development of therapeutic strategies and evaluate their implementation in clinical practice.
3. Relevant Literature and Beyond the State of the Art
Swarm intelligence has emerged as a promising approach for clinical validation in the field of healthcare. Swarm intelligence refers to the collective behavior of decentralized, self-organized systems where individual agents interact with each other to achieve a common goal. In the context of clinical validation, swarm intelligence techniques can be employed to optimize the evaluation of Artificial Intelligence (AI) solutions for non-communicable diseases. Research by [13] demonstrated the effectiveness of a particle swarm optimization algorithm in the clinical validation of AI models for breast cancer detection. The algorithm optimized the selection of features and parameters, resulting in improved accuracy and reduced false positive rates, thereby enhancing the overall performance of the AI solution.
Table 1: Relevant studies to SI methodology
Study | SI Methodology |
[13] | -The methodology involves the use of particle swarm optimization (PSO) as an optimization algorithm. -PSO may be employed to optimize the parameters or features of artificial intelligence models used for breast cancer detection. -The swarm in PSO could represent a population of potential solutions, and the algorithm iteratively refines these solutions to improve the model’s performance. |
[14] | -The methodology incorporates SI techniques, possibly based on algorithms like particle swarm optimization or ant colony optimization. -These swarm techniques could be used to enhance the validation process of deep learning models for cardiovascular disease risk prediction. -The swarm’s collective behavior might help in optimizing and improving the performance of the predictive models. |
[15] | -The methodology involves the application of swarm-based algorithms to enhance the interpretability of AI models used in diabetic retinopathy diagnosis. -Swarm-based techniques could be used to generate insights or explanations for the decisions made by AI models, making them more transparent and interpretable. -The swarm may collectively analyze and interpret the AI model’s outputs to provide meaningful insights for medical professionals. |
Another study by [14] explored the use of swarm intelligence in clinical validation of AI-based systems for cardiovascular disease risk prediction. The study developed a swarm-based evolutionary algorithm that optimized the weights and architecture of a deep learning model. The swarm intelligence approach enabled efficient exploration of the large search space and improved the performance of the AI system. The results demonstrated the potential of swarm intelligence in enhancing the clinical validation process for non-communicable diseases, leading to more accurate and reliable AI solutions.
Swarm intelligence techniques also offer benefits in terms of interpretability and explainability in clinical validation. A study by [15], proposed a swarm-based interpretability framework for AI models used in diabetic retinopathy diagnosis. The framework utilized a swarm optimization algorithm to identify the most important features and generate human-understandable rules to explain the decision-making process of the AI system. This approach not only improved the transparency of the AI solution but also facilitated clinical validation by providing clinicians with insights into how the model arrived at its predictions.
A swarm-based clinical validation framework for AI solutions for non-communicable diseases goes beyond the state of the art by leveraging the collective intelligence of decentralized systems. Unlike traditional validation approaches that rely on manual analysis or single optimization algorithms, swarm intelligence enables the simultaneous exploration of multiple optimization techniques, improving the robustness and performance of the AI models. Furthermore, swarm intelligence techniques offer interpretability and explainability, which are crucial for clinical validation in healthcare settings. By incorporating swarm intelligence, the clinical validation framework can provide clinicians with insights into the decision-making process of AI models, enhancing trust and facilitating the adoption of these solutions in real-world healthcare scenarios. Overall, a swarm-based clinical validation framework represents a significant advancement in the field, bridging the gap between AI research and clinical practice.
4. Methodology
This swarm-based clinical validation framework aims to establish proof of concept and integration procedures within the clinical pathways of healthcare institutes. Inspired by the SI approach, the present paper seeks to facilitate collaboration and communication in the implementation of AI solutions across different sites, effectively creating a collective intelligence. The concept of SI is rooted in the organizational structure observed in natural communities such as bee swarms, ant colonies, flocks of birds, and schools of fish [16]. Individual members of these communities perform simple actions, cooperating with one another, and gradually accumulating their efforts to form a higher-level intelligence that surpasses the capabilities of any individual member [17]. Building upon this concept, the main vision is to introduce a novel validation approach to the healthcare ecosystem that combines the shared features of SI with the fundamental principles of validation approaches and training models. This integration aims to enhance the usability, performance, and safety of AI-based solutions. Furthermore, the vision extends to providing personalized healthcare in the context of disease diagnosis, management, and treatment for patients with NCDs.
Moving from the natural world to the highly interconnected medical realm, the swarm-based framework incorporates several key concepts. Firstly, AI Solutions refer to AI-based systems designed to address specific problems within healthcare. Secondly, within the realm of HealthCare (HC) groups, there exists a diverse ensemble comprising patients, carers, HealthCare Professionals (HCPs), and healthcare datasets. These groups assume pivotal roles in the validation process, with a particular focus on the essential contribution of healthcare datasets. These datasets, typically retrospective in nature, hold paramount importance due to their multifaceted roles:
- Historical Insights: Healthcare datasets encapsulate a rich historical record of patient interactions with the healthcare system. Spanning across years or even decades, they offer profound insights into disease progression, treatment outcomes, and long-term health trends. This historical context serves as a foundational resource, enabling researchers and healthcare professionals to make well-informed decisions.
- Training AI Models: These datasets are fundamental in the development and validation of AI models, including machine learning algorithms designed for disease prediction, medical image analysis, and treatment recommendations. AI models draw upon the vast troves of data contained within these datasets to learn from past cases, thereby enhancing their predictive capabilities and assisting in clinical decision-making.
- Supporting Evidence-Based Medicine: Healthcare datasets are a cornerstone of evidence-based medicine, providing an extensive repository of patient outcomes and treatment responses. Researchers leverage this wealth of data to discern best practices, evaluate treatment efficacy, and identify emerging trends in healthcare. In doing so, they contribute to the evolution of evidence-based healthcare practices and guidelines.
Thirdly, AI solution-specific swarm-based nodes represent instances of the AI solution deployed at specific sites, such as the computing and networking infrastructure of a hospital. Fourthly, the AI solution-specific swarm-based network comprises all the AI solution-specific swarm-based modes and healthcare groups involved in the validation process. Fifthly, the autonomous communication engine serves as a method that guides HC groups to collectively generate results, with their decisions and actions influenced by the overall guidance of the community. This engine establishes the SI network by connecting the AI solution-specific swarm-based node with the healthcare groups participating in the validation process. Lastly, clinical validation evaluation encompasses the assessment of the framework’s effectiveness in clinical validation. Lastly, clinical validation evaluation encompasses the assessment of the framework’s effectiveness in clinical validation (see Figure 1).
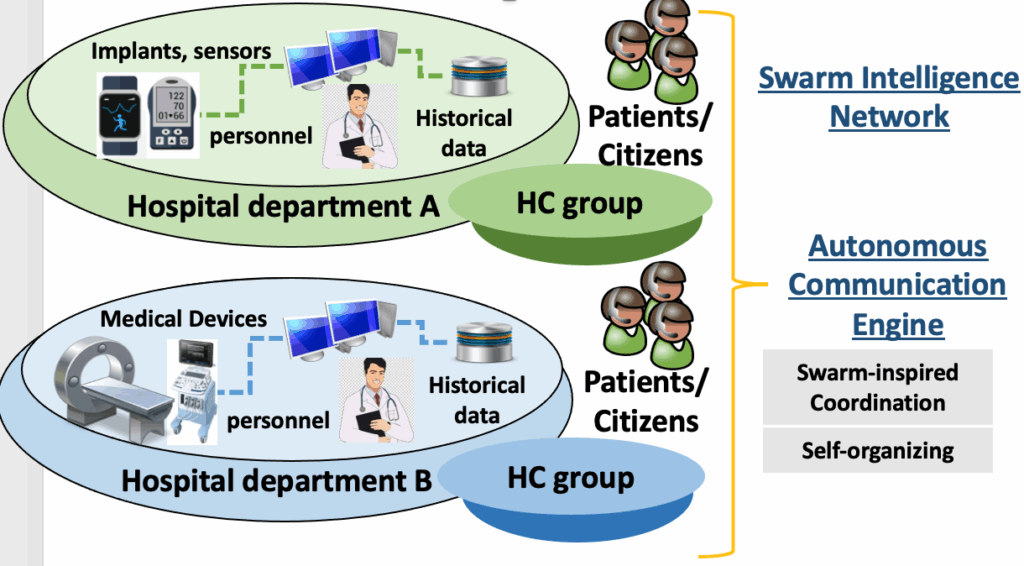
The adopted methodology encompasses evaluation procedures and acceptance criteria aimed at assessing whether an AI Solution meets specified safety, reliability, and quality expectations, thereby enabling its integration into the clinical pathways of healthcare institutes. The proposed swarm-based framework represents a pioneering community-driven approach that harnesses the potential and intellectual resources of healthcare community members. Within this context, HC groups are empowered to operate in a swarm-based manner, each contributing historic patients’ data, information, and knowledge to facilitate effective AI Solution validation. The swarm-based framework aims to foster this novel form of collaboration, drawing inspiration from SI, and utilizing an autonomous communication engine. This engine enables autonomic deployment, cluster formation, and hierarchical communication within the healthcare community. By applying principles from bio-inspired computing, such as decentralized knowledge exchange, advanced self-organized networks/systems design, and self-functionality support, the engine enhances the accuracy and coordination of HC groups. Consequently, the proposed framework effectively harnesses the collective intelligence of diverse HC groups, streamlining the development, implementation, and evaluation of a pan-European clinical validation framework. Lastly, a comprehensive evaluation methodology is proposed, encompassing multiple perspectives, including analytical validation, clinical utility, security aspects, behavioural economics, and integration pathways.
5. Swarm Intelligence Network Establishment and Capabilities
The key innovation lies in the capabilities that the SI network can achieve, surpassing what individual installations of the AI solution can accomplish. The following list outlines the advancements of the AI Solution-Specific Swarm-based Node over the local deployment of the AI solution, as well as the unique capabilities of the connected swarm-based node:
Internal Evaluation: The AI Solution-Specific Swarm-based Node can perform internal evaluations of its model using available HC groups. It can assess metrics such as accuracy, sensitivity, and F-score to determine if it meets the required standards. This ability enables the node to “know that it does not know,” a crucial aspect for trustworthy AI systems. Additionally, if multiple models are available, the swarm-based node can evaluate and select the model that best meets the required criteria. For systems with Continuous Learning, the node can track older models and assess if they better fit the current data than more recent models.
Model Exchange: In networked swarm-based nodes, a node has the capability to exchange models with other nodes and test them against its own data. This process allows the node to identify external models that may fit its data better, potentially due to similarities between patient populations or the availability of more extensive data on the other site.
Autonomous Communication Engine: The proposed networks rely on an autonomous communication engine that supports a distributed network of autonomous nodes collaborating to build AI knowledge in a manner that suits each node. This framework enables improved service delivery to patients at a pan-European or global scale.
Privacy Considerations: Privacy concerns make it challenging to share healthcare data from HC groups. The use of solutions such as Federated Learning or Swarm Learning offers practical options for aggregating knowledge from data without sharing the raw data.
This approach introduces capabilities that are not currently found in isolated or federated learning AI systems, presenting new avenues for research. Specifically, the SI approach can help address issues related to site biases and contribute to the detection of emerging data quality problems in one or more nodes. Moreover, there is potential to repurpose explainable AI methods to provide explanations not only for AI predictions but also for trends in patient data, the Swarm-based node’s model selection decisions, and the network as a whole.
6. Evaluation Methodology
This section presents the main layers of the methodology that will be utilized to evaluate AI solutions and their corresponding AI Solution-Specific Swarm-based Nodes from multiple perspectives. The proposed methodology encompasses five (5) key layers, which are described below and visualized in Figure 2:
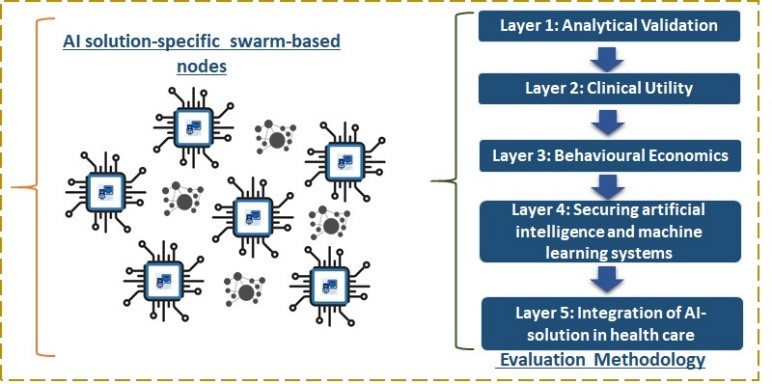
6.1. Layer 1: Analytical Validation
Layer 1 focuses on the analytical validation and fine tuning of collected data from hospitals, as well as the training and utilization of AI models. This layer consists of three main steps.
The first step involves the collection, analysis, and modelling of historical patient data from each hospital. The collected data will be validated, checking for missing values and erroneous measurements. Data augmentation techniques will be employed when necessary to enrich and balance the data samples, aiding in model building. Additionally, appropriate data summarization and feature representations will be utilized to smooth variables with high variance caused by noisy measurements or missing values. It’s worth noting that patient data involved in this step will undergo pseudonymization using techniques such as (k, km)-anonymity and LKG-privacy, which are methods designed to protect patient privacy and confidentiality.
Following the principles of Federated and Swarm Learning, the data will be stored, pre-processed, validated, and augmented locally. Model training and validation steps will be performed both locally and in a distributed manner. Specifically, ML models will be trained locally for each data node (hospital), while Swarm nodes will handle aggregating and exchanging locally learned parameters in a distributed fashion using blockchain technology.
The second step involves internal validation of the AI models and evaluating their performance using labelled data samples, such as electronic health records of specific patient cohorts. Techniques employed here include standard k-fold cross-validation and Monte-Carlo cross-validation. The goal of internal validation is to ensure that using locally learned model parameters achieves competitive trade-offs in terms of predictive performance when provided with new, unseen data samples from similar patient cohorts within the same hospital node.
The third and most critical step entail external validation. In this step, the locally learned models from the first step are applied to other hospital nodes via the HC network using new data samples from different hospitals and patient cohorts. The objective here is to validate the utility and performance of Swarm learning by leveraging the shared parameters from locally trained models, regardless of the originally collected data. Moreover, the AI models will be enhanced with explainability capabilities, enabling them to provide not only accurate predictions but also explanations for those predictions. Explainability methods, such as LIME and SHAP, will be employed and extended to assess feature importance, which is a key component of building final model explanations. Initially, these explainability methods will be applied locally and subsequently shared and orchestrated in a distributed manner. Feature importance values, similar to the ML model values, will be shared among network nodes. The clinical validity of these explanations will be evaluated using standard explainability metrics, saliency and feature visualization methods, exemplar methods, and counterfactuals. The aim is to demonstrate that models with high explainability are preferable over opaque models.
6.2. Layer 2: Clinical Utility
Layer 2 aims to progress beyond the analytical validation and performance assessment of AI algorithms. This layer focuses on integrating the algorithms into the workflows of HCPs and ensuring the acceptance and utilization of the derived AI information. The primary objective is to achieve clinical utility of the AI models by improving clinical outcomes and achieving prophylactic or therapeutic goals through the “output-action” pairing model (OAP). A systematic assessment will be conducted for each AI outcome to determine how it can act upon or mitigate the output to enhance medical care. Ethical considerations, including regulatory requirements, bias and fairness, representation bias, historical bias, FAIR principles, and WHO guidance for ethics and governance of AI in health, will be carefully addressed in this layer.
Clinical utility will involve engaging different groups of patients, caregivers, and HCPs to ensure that the validated AI models can be integrated into their practices and incorporated into appropriate clinical pathways. Before commencing the operational phase of the project, various preparatory measures will be implemented to ensure that the pilot sites are fully equipped to embrace the AI solutions. These measures involve employing co-production approaches, such as early engagement with healthcare providers (HCPs) and stakeholders, delivering comprehensive training to end-users and stakeholders participating in the pilots, as well as localizing, adapting, installing, and deploying the AI solutions across multiple consortium operators. During this stage, test cases will be formulated to evaluate all functionalities of the AI solutions, compile best practices that can be useful to healthcare institutions, authorities, and standardization bodies, and gather input from real-life experiments. The clinical utility will involve testing, validation, and evaluation of the AI solutions from functional, technical, standardization, and business perspectives.
Stakeholders such as HCPs, hospitals, and various healthcare teams will be involved under the guidance of technological partners applying co-production methodologies. The evaluation outcomes will be analyzed to develop best practices for the wider applicability and use of the AI solutions and the associated collaborative approach. These best practices will aim to generalize the approach for use in NCDs throughout the dynamic healthcare supply chain. Empowering and fostering collaboration among stakeholders through co-production is crucial for the development of sustainable AI. Therefore, active engagement with stakeholders will be prioritized, ensuring that they thoroughly scrutinize the project through sound policy and government monitoring to ensure its adequateness and sustainability. Continuous scrutiny is essential as sustainable AI encompasses the entire life cycle of AI, including design, training, development, validation, re-tuning, implementation, and use.
6.3. Layer 3: Behavioural Economics
Layer 3 will focus on assessing and establishing the cost-effectiveness of AI solutions. This will be achieved through appropriate cost-effectiveness analysis and full or partial economic evaluations, using methods such as the CHEERS checklist or Assessing Cost-Effectiveness (ACE) methodology. This layer will systematically explore various time points to assess the economic credentials of the AI solutions and facilitate evidence-based decision-making across all pilot sites.
A cost-minimization analysis will be conducted to evaluate the potential savings of the AI solutions compared to the current human healthcare delivery. Economic modelling studies will be performed, utilizing patients with NCDs. Methods such as decision tree models or TreeAge Pro will be used to compare the actual cost of using HCPs with the estimated cost of using the AI solutions. Model parameters, including NCDs prevalence rates, treatment and care costs, cost of medical consultations, and diagnostic performance (such as sensitivity and specificity), will be incorporated into each model. The primary outcome of the analysis will be the total cost for each model. Deterministic sensitivity analyses will also be conducted to assess the sensitivity of the results to key model assumptions.
Overall, evaluating the cost-effectiveness of these population-based AI solutions will support clinical decision-making in treatment and care, providing a strong economic rationale. The overarching goal of this layer is to provide robust evidence regarding the effectiveness, affordability, feasibility, and acceptability of the newly developed protocol. Additionally, the assessment will consider equity impact and sustainability factors, ensuring that AI solutions are equitable and sustainable in their implementation.
6.4. Layer 4: Securing artificial intelligence and machine learning systems
This layer introduces an AI model-driven risk assessment approach aimed at identifying and measuring relevant threats and vulnerabilities. It will estimate the potential impacts, considering the abundance and sensitivity of healthcare data processed by the AI solutions. The main outcome of this process will be a comprehensive defense strategy that incorporates security-by-design countermeasures, such as adversarial training and robust optimization. Additionally, methods for protecting the training datasets, such as data sanitization techniques, will be employed. These measures aim to assist the proposed AI and ML models in building robust defences against various attacks during both test and training times.
6.5. Layer 5: Integration of AI-solution in health care
A critical challenge in implementing AI solutions in healthcare is the integration into existing complex clinical workflows and care pathways. To address this, we propose embedding AI tools directly into Electronic Health Record (EHR) systems. Specifically, for AI-enabled risk prediction models, seamless integration into EHRs will not only enhance clinicians’ performance but also simplify and streamline the clinical workflow, supporting clinical decision-making at the point of care and transforming them into “systems of intelligence” and “systems of engagement.”
Standardization and interoperability are essential elements for successful integration due to the multiple components involved in typical clinical workflows. Since data is collected through various methods for different purposes and can be stored in different formats using diverse database and information systems, it is crucial to standardize them into a common format to effectively utilize AI-based technologies. We will employ a set of standards to establish a consistent nomenclature, facilitating uniform methods of data storage and retrieval. These standards enable seamless integration of AI tools with EHRs, ensuring compatibility in terms of user interface and patient data. Examples of proposed standards include SMART on FHIR (Substitutable Medical Applications and Reusable Technologies on Fast Healthcare Interoperability Resources framework), which is based on the Health Level 7 (HL7) framework of standards for electronic health information exchange, as well as DICOM and HITECH.
To maximize alignment among clinical workflow, evidence-based clinical standards of care, and practice patterns for quality of care, we will employ social innovation approaches. These approaches involve giving a voice to relevant stakeholders, including patients, caregivers, and healthcare professionals, to express their views and establish preferred practice patterns. By incorporating the perspectives and insights of these stakeholders, we can ensure that the integration of AI solutions aligns with the needs and expectations of the healthcare community.
7. Practical Implications of the Swarm-Based Framework
AI has gained significant attention in the healthcare industry for its potential to revolutionize the diagnosis, treatment, and management NCDs. However, the successful integration of AI solutions into clinical practice requires robust clinical validation frameworks. This paper explores the practical and clinical implications of a swarm-based clinical validation framework for AI solutions targeting NCDs. Clinical validation involves assessing the performance, safety, and effectiveness of AI solutions in real-world clinical settings. It ensures that the AI algorithms produce accurate and reliable results, ultimately benefiting patients and healthcare providers. Traditional validation approaches, such as retrospective studies and randomized controlled trials, have limitations in terms of cost, time, and generalizability. The developed swarm-based clinical validation framework offers a promising alternative. The proposed framework leverages the collective intelligence of a swarm, which comprises multiple independent AI models trained on diverse datasets.
Practical implications of this framework include:
Enhanced Accuracy and Reliability: One of the key practical benefits of the swarm-based clinical validation framework is the enhanced accuracy and reliability of AI solutions. By leveraging the collective intelligence of multiple independent AI models, the framework mitigates the risk of biased or erroneous outcomes that may arise from individual models. The diverse expertise and perspectives within the swarm contribute to more robust predictions, leading to improved diagnostic accuracy, disease prognosis, and treatment recommendations [18].
Generalizability: A significant challenge in AI development is the generalizability of models across diverse patient populations and healthcare settings. The swarm-based approach addresses this challenge by aggregating data from multiple institutions, representing a broader spectrum of patients and clinical practices. This diversity enhances the generalizability of AI solutions, making them applicable across different demographics, geographical regions, and healthcare systems. It reduces the risk of overfitting to a specific dataset, thereby improving the practical utility and relevance of AI models [19].
Knowledge Sharing and Collaboration: The framework fosters knowledge sharing and collaboration among participating institutions. By pooling together diverse datasets and expertise, the framework enables the exchange of best practices, research findings, and clinical insights. This collaboration benefits all participants, as it facilitates the collective development of AI models that are more accurate, reliable, and effective in addressing NCDs. The shared knowledge also promotes advancements in the field and encourages the adoption of standardized protocols and guidelines [8].
Cost and Time Efficiency: Traditional validation approaches, such as retrospective studies or randomized controlled trials, can be time-consuming, costly, and resource intensive. In contrast, the proposed framework optimizes resource utilization by distributing the validation process among multiple institutions. This approach significantly reduces the time required for validation and lowers the associated costs. By sharing the workload, institutions can collaborate on large-scale validation studies without imposing excessive burdens on any single entity. The resulting efficiency expedites the translation of AI solutions from research to clinical practice, benefiting patients and healthcare providers alike [20].
Ethical Considerations: Data privacy and ownership are critical ethical considerations in AI research and development. The swarm-based clinical validation framework addresses these concerns by enabling collaborative validation without the need for sharing sensitive patient data. Instead, participating institutions contribute their trained models to the swarm, while the validation process focuses on refining the collective decision-making process rather than accessing individual patient data. This approach ensures compliance with privacy regulations and promotes responsible use of AI technologies in healthcare [21].
The practical implications of the swarm-based clinical validation framework for AI solutions targeting non-communicable diseases are significant. By enhancing accuracy, improving generalizability, fostering collaboration, optimizing resource utilization, and addressing ethical considerations, this framework holds great promise for the successful integration of AI into clinical practice. It paves the way for more reliable, efficient, and effective AI solutions that can significantly impact the management and treatment of NCDs.
8. Clinical Implications of the Swarm-Based Framework
This paper also explores the clinical implications of the swarm-based clinical validation framework for AI solutions targeting NCDs. In particular:
Enhanced Diagnostic Accuracy: Precise and timely diagnosis constitutes a cornerstone of effective non-communicable disease (NCD) management. The swarm-based clinical validation framework elevates diagnostic precision by capitalizing on the collaborative intelligence of numerous independent AI models. The breadth of models integrated into the swarm bolsters prediction robustness, thereby reducing diagnostic inaccuracies and furnishing healthcare professionals with heightened confidence in diagnostic assistance [21].
This enhancement in diagnostic precision is instrumental in ensuring that patients receive accurate assessments of their NCDs, facilitating more targeted and effective treatment strategies. Moreover, the collective approach of the swarm-based framework fosters adaptability to evolving medical data and insights, further solidifying its utility in modern healthcare practices.
Personalized Treatment Recommendations: NCDs often require individualized treatment approaches. The swarm-based framework enables personalized treatment recommendations by considering a wide range of clinical data from diverse patient populations. The collaboration among institutions within the swarm facilitates the identification of optimal treatment strategies tailored to individual patient characteristics, leading to improved treatment outcomes [8].
Disease Prognosis and Risk Assessment: Accurate prognosis and risk assessment are vital for managing NCDs and preventing complications. The swarm-based framework enhances the accuracy of disease prognosis by aggregating and analyzing data from multiple institutions. This collaborative approach enables the development of predictive models that can assess disease progression, evaluate treatment response, and identify individuals at high risk of developing complications [22].
Clinical Decision Support: AI solutions validated through the swarm-based framework provide valuable clinical decision support to healthcare professionals. By leveraging the collective knowledge and expertise of the swarm, these solutions can assist in evidence-based decision-making, treatment planning, and monitoring of NCDs. This support enhances clinical workflow efficiency, reduces variability in care, and improves patient outcomes [19].
Improved Patient Engagement and Education: Effective management of NCDs requires active patient engagement and education. The swarm-based framework can contribute to patient engagement by providing personalized health information, educational resources, and self-management tools. AI-powered applications developed within the framework can empower patients to actively participate in their own care, leading to better adherence to treatment plans and lifestyle modifications [23].
Real-time Monitoring and Early Intervention: The proposed framework facilitates real-time monitoring and early intervention for NCDs. AI solutions integrated into remote monitoring systems can continuously analyze patient data, detect subtle changes, and alert healthcare providers to potential risks or worsening conditions. This enables timely interventions, reducing the burden of hospitalizations and improving patient outcomes [24].
Continuous Learning and Improvement: The swarm-based framework promotes continuous learning and improvement of AI models. As new data and clinical insights become available, the swarm can adapt and refine the models to incorporate the latest knowledge. This iterative process ensures that AI solutions remain up to date, accurate, and aligned with evolving clinical practices and guidelines [25].
The clinical implications are evident and crucially important, by enhancing diagnostic accuracy, enabling personalized treatment recommendations, improving disease prognosis, providing clinical decision support, fostering patient engagement, facilitating real-time monitoring, and promoting continuous learning, this framework holds great promise for improving NCD management and patient outcomes.
9. Conclusions and Future Work
The swarm-based clinical validation framework for AI solutions targeting NCDs has demonstrated several significant advantages and promising outcomes. By leveraging the collective intelligence of multiple independent AI models, this framework enhances diagnostic accuracy, enables personalized treatment recommendations, improves disease prognosis, provides clinical decision support, fosters patient engagement, facilitates real-time monitoring, and promotes continuous learning. These practical and clinical implications hold great potential for transforming NCD management and improving patient outcomes.
The swarm-based approach mitigates the limitations of individual AI models, such as bias, overfitting, and generalizability issues. By aggregating data from diverse patient populations and healthcare settings, the framework improves the generalizability and robustness of AI solutions. Moreover, the collaboration and knowledge sharing among institutions within the swarm foster advancements in the field, encourage standardized protocols, and enhance the overall quality of AI models. The framework also addresses ethical considerations by ensuring data privacy and promoting responsible use of AI technologies. By focusing on refining the collective decision-making process rather than accessing individual patient data, it maintains compliance with privacy regulations and promotes transparency and accountability in AI-driven healthcare.
While the swarm-based clinical validation framework shows promise, further research and development are required to maximize its potential and address remaining challenges. There are several suggested areas which would require future work:
Integration into Clinical Workflows: Efforts should be made to seamlessly integrate AI solutions validated through the swarm-based framework into existing clinical workflows. Integration should consider usability, interoperability, and the ability to integrate with electronic health record systems. User feedback and iterative improvement should guide the development process.
Real-world Implementation Studies: Conducting large-scale implementation studies is crucial to evaluate the real-world impact of swarm-based AI solutions. These studies should assess clinical outcomes, patient satisfaction, cost-effectiveness, and provider acceptance to demonstrate the value of the framework in routine clinical practice.
Validation in Diverse Populations: It is essential to validate swarm-based AI models across diverse populations to ensure equitable and unbiased healthcare. Efforts should be made to include underrepresented groups and marginalized populations in validation studies to avoid exacerbating existing health disparities.
Ethical Considerations and Governance: Continued attention should be given to ethical considerations in AI development and deployment. Clear guidelines and governance frameworks should be established to address issues such as transparency, accountability, explainability, and algorithmic bias. Collaboration between healthcare professionals, researchers, policymakers, and ethicists is essential in shaping responsible and ethical AI practices.
Long-term Monitoring and Evaluation: Continuous monitoring and evaluation of swarm-based AI solutions are necessary to assess their long-term impact, address emerging challenges, and ensure ongoing improvement. Longitudinal studies and feedback mechanisms from users and stakeholders can provide valuable insights for refinement and optimization.
By pursuing these avenues of future work, the swarm-based clinical validation framework can advance the field of AI in NCD management, driving innovation, improving patient outcomes, and contributing to the transformation of healthcare delivery. In conclusion, the swarm-based clinical validation framework of AI solutions for non-communicable diseases offers practical and clinical implications that have the potential to revolutionize healthcare. While further research and development are needed, this framework holds significant promise for improving diagnostic accuracy, treatment personalization, disease prognosis, clinical decision support, patient engagement, and real-time monitoring. By addressing ethical considerations and embracing ongoing improvement, this framework can pave the way for more effective and equitable healthcare delivery in the future.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgment
The research conducted in this paper was triggered by the European Union’s Horizon Research and Innovation Project, “IMPlementing geriatric assessment for dose Optimization of CDK 4/6-inhibitors in older bReasT cAncer patieNTs (IMPORTANT)” under Grant Agreement No. 101104589. Also, this work has been supported by the project ‘A Dynamic and Self-Organized Artificial Swarm Intelligence Solution for Security and Privacy Threats in Healthcare ICT Infrastructures’ (AI4HEALTHSEC) under grant agreement No 883273. The project was funded by the European Union’s Horizon 2020 research and innovation programme. The authors are also grateful for the financial support provided by the European Union’s Digital Europe Programme (DEP) Project, ‘Collaborative, Multi-modal and Agile Professional Cybersecurity Training Program for a Skilled Workforce In the European Digital Single Market and Industries’ (CyberSecPro) under Grant Agreement No. 101083594. Special thanks to the consortiums of these projects and their contributions. The views expressed in this paper represent only the views of the authors and not of the European Commission, or the partners in the above-mentioned projects, or University of Essex, or University of Piraeus, or University of Brighton, or trustilio B.V, or Security Labs Consulting Limited.
- World Health Organization. “Noncommunicable diseases”, 2021, Retrieved from https://www.who.int/health-topics/noncommunicable-diseases#tab=tab_1
- M. Marmot, M, “Health equity in England: The Marmot Review 10 Years on”, BMJ, vol. 693, no. 1, pp. 1-20, 2020, doi:10.1136/bmj.m693
- F. Luna, V.A. Luyckx, “Why have Non-communicable Diseases been Left Behind?” Asian Bioeth Rev, Vol. 12, no.1, pp. 5-25, 2020, doi: 10.1007/s41649-020-00112-8
- N.T. Castillo-Carandang, R.D. Buenaventura, Y.C. Chia, D. Do Van, C. Lee, N.L. Duong, et al., “Moving Towards Optimized Noncommunicable Disease Management in the ASEAN Region: Recommendations from a Review and Multidisciplinary Expert Panel”, Risk Manag Healthc Policy, Vol. 13, no. 1, pp. 803-819, 2020, doi: 10.2147/RMHP.S256165. PMID: 32765135; PMCID: PMC7371561.
- S. Xiong, H. Lu, N. Peoples, et al., “Digital health interventions for non-communicable disease management in primary health care in low-and middle-income countries”, npj Digit Med, Vol. 6, no. 12, pp. 1-20, 2023, doi: https://doi.org/10.1038/s41746-023-00764-4
- Z. Obermeyer, E.J. Emanuel, L.O. Gostin, “Big data, big responsibilities: A paradox of information in the era of artificial intelligence”, JAMA, Vol. 316, no. 6, pp. 601–602, 2016.
- Α. Rajkomar, J. Dean, I. Kohane, “Machine learning in medicine”, New England Journal of Medicine, Vol. 380, no. 14, pp. 1347-1358, 2018, doi: 10.1056/NEJMra1814259.
- E.J. Topol, “High-performance medicine: The convergence of human and artificial intelligence”, Nature Medicine, Vol. 25, no. 1, pp. 44-56, 2019, doi: 10.1038/s41591-018-0300-7.
- K. Denecke, “Artificial intelligence and medical informatics: State of the art and challenges for the future”, Yearbook of Medical Informatics, Vol. 28, no. 1, pp. 164-166, 2019, doi: 10.1055/s-0039-1677902.
- X. Liu, L. Faes, A.U, Kale, S.K. Wagner, D. Fu, et al., “A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: a systematic review and meta-analysis”, The Lancet Digital Health, Vol. 2, no. 6, pp. 271-297, 2020, doi: 10.1016/S2589-7500(20)30034-7.
- G. Hinton, O. Vinyals, J. Dean, Distilling the knowledge in a neural network. 2019, arXiv preprint arXiv:1503.02531.
- G. Huang, Z. Liu, L. Van Der Maaten, K.Q. Weinberger, “Densely connected convolutional networks”, In Proceedings of the IEEE conference on computer vision and pattern recognition, pp. 4700-4708, 2018.
- R.S. Parpinelli, T.O. Sousa, P.E. Miyagi, “Particle swarm optimization applied to the clinical validation of artificial intelligence models for breast cancer detection”, Expert Systems with Applications, Vol.155, no. 1, pp. 113-134, 113434, 2020.
- W. Dai, Y, Ma, Y. Xiong, Y. Li, Y., H. Zhang, X. Zhang, “A swarm intelligence-based clinical validation approach for cardiovascular disease risk prediction using deep learning models”, Computers in Biology and Medicine, Vol. 137, no 1, pp. 1-24, 2021, doi: 10.1016/j.compbiomed.2021.104819.
- H.D. Nguyen, M.D. Nguyen, T.H. Tran, D.A. Duong, “A swarm-based interpretability framework for AI models in diabetic retinopathy diagnosis”, Expert Systems with Applications, Vol: 189, no. 1, pp. 115-134, 2021.
- J. Kennedy, R. Eberhart, R. Swarm Intelligence. Morgan Kaufmann, 2001.
- E. Bonabeau, M. Dorigo, G. Theraulaz, “Swarm Intelligence: From Natural to Artificial Systems”, Oxford University Press, 1999.
- G. Hinton, et al., “Deep neural networks for acoustic modeling in speech recognition: The shared views of four research groups”, IEEE Signal Processing Magazine, Vol. 29, no. 6, pp. 82-97, 2015, doi: 10.1109/MSP.2012.2205597.
- A. Esteva, et al., “Dermatologist-level classification of skin cancer with deep neural networks” Nature, Vol. 542, no. 7639, pp. 115-118, 2017, doi: 10.1038/nature21056.
- J. Lee, et al., “Machine learning in medical imaging: A systematic review of the literature and potential applications for emergency radiology”, Emergency Radiology, Vol. 27, no. 6, pp. 669-679, 2020, doi: 10.1007/s10140-020-01860-w.
- A. Rajkomar, et al., “Scalable and accurate deep learning with electronic health records”, npj Digital Medicine, Vol. 1, no. 1, pp. 18-24, 2018, doi: 10.1038/s41746-018-0029-1.
- A.L. Beam, et al., “Big data and machine learning in health care”, JAMA, Vol. 322, no. 13, pp. 1317-1318, 2020, doi: 10.1001/jama.2019.21445.
- Z. Obermeyer, et al., “Dissecting racial bias in an algorithm used to manage the health of populations”, Science, Vol. 366, no. 6464, pp. 447-453, 2019, doi: 10.1126/science.aax2342.
- F.S. Collins, et al., “A new initiative on precision medicine”, New England Journal of Medicine, Vol. 372, no. 9, pp. 793-795, 2020, doi: 10.1056/NEJMp1500523.
- K.H. Yu, et al., “Artificial intelligence in healthcare”, Nature Biomedical Engineering, Vol. 2, no. 10, pp. 719-731., 2018, doi: 10.1038/s41551-018-0305-z.
- Kitty Kioskli, Spyridon Papastergiou, Theofanis Fotis, “Conceptual Business Model Framework for AI-based Private 5G-IoT Networks”, Journal of Engineering Research and Sciences, vol. 3, no. 10, pp. 13–20, 2024. doi: 10.55708/js0310002
- Kitty Kioskli, Spyridon Papastergiou, Theofanis Fotis, “Blockchain Based Framework for Securing Students’ Records”, Journal of Engineering Research and Sciences, vol. 1, no. 6, pp. 45–54, 2022. doi: 10.55708/js0106006
- Kitty Kioskli, Spyridon Papastergiou, Theofanis Fotis, “Analytical Framework to Minimize the Latency in Tele-herbal Healthcare Service”, Journal of Engineering Research and Sciences, vol. 1, no. 1, pp. 39–50, 2022. doi: 10.55708/js0101004

