Isolation and Characterization of the Bioplastic Producing Bacteria Using Low-Cost Substrate, Sawdust
Journal of Engineering Research and Sciences, Volume 2, Issue 12, Page # 7-14, 2023; DOI: 10.55708/js0212002
Keywords: Bioplastics, PHB production, Agri wastes-based bioplastics
(This article belongs to the Section Biotechnology and Applied Microbiology (BAM))
Export Citations
Cite
Javaid, A. , Aslam, S. , Qaisar, H. , Batool, F. , Javed, R. and Qaisar, M. W. (2023). Isolation and Characterization of the Bioplastic Producing Bacteria Using Low-Cost Substrate, Sawdust. Journal of Engineering Research and Sciences, 2(12), 7–14. https://doi.org/10.55708/js0212002
Anam Javaid, Sumaira Aslam, Hira Qaisar, Farhat Batool, Rimsha Javed and Muhammad Waqas Qaisar. "Isolation and Characterization of the Bioplastic Producing Bacteria Using Low-Cost Substrate, Sawdust." Journal of Engineering Research and Sciences 2, no. 12 (December 2023): 7–14. https://doi.org/10.55708/js0212002
A. Javaid, S. Aslam, H. Qaisar, F. Batool, R. Javed and M.W. Qaisar, "Isolation and Characterization of the Bioplastic Producing Bacteria Using Low-Cost Substrate, Sawdust," Journal of Engineering Research and Sciences, vol. 2, no. 12, pp. 7–14, Dec. 2023, doi: 10.55708/js0212002.
Plastics are routinely used in the packaging of materials as well as in industrial production. However, once in the environment, they are non-biodegradable, posing severe threats to the ecosystems. Bioplastic replaces conventional plastic, which is biodegradable due to its biological origin and does not affect environment. Sawdust is a very important agro-waste screened as a substrate for bioplastic production. In the present study, bacteria capable of producing bioplastic by utilizing sawdust as a cheap substrate were isolated and optimized for bioplastic production. Eight sawdust utilizing bacteria were isolated and the strains were designated as SD1 to SD8. These indigenous bacterial isolates were screened for bioplastic production using Sudan Black B Staining. Among the bacterial isolates, the bioplastics (PHB) production levels were 0.046 g/mL to 0.32 g/mL, while the maximum PHB (g/ml) production (0.32 ± 0.008g/mL) was given by SD2 isolate identified as the Bacillus cereus-SD2 strain through 16SrDNA sequencing. The isolate SD2 was optimized for bioplastic production at different growth conditions. The best temperature for the bioplastic production was 37˚C in the saw dust containing low-cost medium and yielded 0.32 ± 0.00 optical density at wavelength 235 nm of crotonic acid. The isolate SD2 showed a higher PHB (g/ml) yield of 0.31 ± 0.008 under alkaline conditions of pH 9. Sufficient oxygen was required for the higher PHB (g/ml) production by the bacterial isolate SD2, which yielded a 0.32 ± 0.006 level of PHB as compared to the non-aeration. The Bacillus cereus-SD2 is a promising bacterial which can produce environmentally friendly bioplastics using low-cost substrate. Finding more growth condition for enhanced bioplastics yields in future are suggested to scale up the production process at industrial level.
1. Introduction
Plastic, a type of polymer, has influenced every part of the modern world mainly because of its lightweight, low cost, robustness and flexibility [1]. In recent COVID -19 pandemic the use of plastic increased in medical fields and disposable packaging [2]. They are mostly made from carbon atoms derived from fossil fuels [3]. A range of additives or plasticizers are also used in their production. Along with the scores of benefits associated with plastics, the plastic debris and plasticizers discharged from them have become an environmental hazard today [4]. Most of the plastics, particularly the petroleum-based are highly resistant to biodegradation, resulting in negative impacts on the physical and biological aspects of the environment [5]. One of the sustainable and futuristic solutions of this issue is the development of bioplastics.
Bioplastics are the materials synthesized by deriving all carbon from renewable feedstock. Owing to their biodegradable nature, they can substitute for conventional plastics. They are made up of monomeric chains that are linked by ester bonds, also known as polyesters. Among many types and different degrees of biodegradability, polyhydroxyalkonate (PHA) and polyhydroxybutyrate (PHB) are very common bioplastics. They are produced by a variety of bacteria as energy reserves under deficient conditions [6]. Polyhydroxybutyrate (PHB) potential candidate can also be obtained by microbial fermentation and is intensively researched [7-8]. The PHB market is predicted to reach $98 million by 2024 with a compound annual growth rate of 11.24% [9].
However, as eco-friendly alternatives to synthetic plastics, the major limiting factor in the wider adaptability of bioplastics is the expensive carbon substrate used in the fermentation process [10]. As a biodegradable polymer with characteristics resembling those of conventional plastics, polyhydroxyalkanoate (PHA) is seen as a potential replacement for plastics made from petrochemical derivatives. Hundreds of bacteria can reportedly store PHA in prokaryotic cells to sustain their own development and metabolism [11]. Paddy straw is one of the agricultural wastes produced in large quantities during the rice harvest [12] Wide variety of cost-efficient substrates, such as wheat bran, rice bran, ragi bran, jambul seed powder and orange were screened for PHA production. In numerous studies sawdust was also employed to produce PHA [13-14]. It could be potentially inexpensive and renewable feedstock for production of PHA as the woodworking industry generates this solid waste.
The primary factor restricting the large-scale manufacture of PHAs at the moment is the higher production cost. According to various polymer types, the cost of producing PHAs has increased to 4,000–15,000 US$/Mt, which is roughly 4–10 times more expensive than petrochemical-based polymers [15]. Thus, recent studies have aimed to utilize low-cost feedstocks such as agricultural, food processing, and municipal wastes [16]. Therefore, in present study, we demonstrated the production of PHB from saw dust isolates as low-cost feedstock.
2. Methodology
2.1. Sampling
The bioplastic-producing bacteria were isolated from the soil samples collected from industrial area of the city Kasur (31.12° North latitude and 74.45° East longitude), Pakistan and placed in a sterile glass bottle under standard sterilization conditions. The samples were brought to the Microbiology and Biotechnology Laboratory of Zoology Department, GC Women University, Faisalabad and were stored in the fridge at 4°C for later use.
2.2. Enrichment of sawdust substrate utilizing bacteria
The substrate media was prepared by adding 2 g of sawdust in flask, containing 50 mL of distilled water. This flask containing substrates was then autoclaved at 121ºC and 15 lbs. pressure for 15 minutes. After autoclaving, when flasks containing the substrates reached room temperature, 1 g of soil sample was added to each of the flasks. They were then incubated for 48 h at room temperature.
2.3. Isolation and characterization of the bacteria
A specific volume of the enrichment culture was spread with a sterilized glass spreader over the nutrient agar plate. The agar plates were incubated at 37ºC for 24 h and growth of the bacterial isolates was observed and recorded. The bacterial colonies were processed for pure culturing and morphological and physicochemical characteristics of the bacterial isolates were determined [17].
2.4. Identification of the bacteria by 16SrRNA
The select bacterial isolate was characterized through 16S rDNA sequencing. To achieve this, DNA was extracted from a bacterial culture that had been grown overnight in nutrient broth at 37°C, following the method outlined by Li et al. [18]. For the PCR amplification of the 16S rDNA, the following primers were utilized: 27f (5′-AGATTTGATCMTGGCTCAG-3′) and 1492r (5′-GGTTACCTTGTTACGACTT-3′). The PCR reaction mixture comprised the DNA extract, MgCl2, dNTPs, forward and reverse primers, DNA Taq polymerase, and Taq buffer. The PCR process involved an initial denaturation step at 94°C for 3 minutes, followed by 35 cycles of denaturation at 95°C for 30 seconds, an annealing step at 60°C for 2 minutes, and an extension step at 72°C for 1 minute. A final extension step was carried out at 72°C for 30 minutes using a thermal cycler (Hamburg 22331, Germany). The resulting PCR product consisted of amplified bands measuring 1.5 kb, which were subsequently subjected to electrophoresis and visualized under UV light [Gel Doc, Bio-Rad Laboratories, USA]. To purify the DNA bands, a Gene Purification Kit (Fermentas) was employed, following the manufacturer’s instructions. Subsequently, sequencing was performed using Big Dye Terminator v3.1 cycle sequencing ready reactions (Macrogen, Korea) at the DNA Sequencing Facility in Korea. The 16S rRNA gene homology search was conducted using BLAST (http://www.ncbi.nlm.nih.gov/BLAST/). The 16S rDNA sequence obtained for the SD2 isolate in this study was submitted to GenBank to obtain an accession number.
2.5. Selection of the Polyhydroxy butyrate (PHB) producing bacteria
The bacterial isolates from the agar slants were revived in nutrient broth. Then these isolates were screened for PHB production following the Sudan Black B staining method [6]. Sudan Black B stain (0.3%) was prepared in 70% ethanol. Then a bacterial smear was prepared. For this purpose, one loop full of bacterial colonies was picked with the help of a sterilized loop and placed in a small drop of sterilized distilled water on a clean glass slide. The smear was prepared with the help of a clean toothpick. Later, the smear was heat fixed. Few drops of Sudan Black B stain solution were poured over the smear and incubated for 10 minutes. Then the slide was washed with distilled water and counter-stained with safranin and left for 5 minutes. The prepared slide was observed under a microscope and the results were noted. All bacteria with PHB production ability were then chosen for further research.
2.6. Preparation of Fermentation Media
The N-limited medium [19] consisting of (NH4)2SO4 – 2 g/L; KH2PO4 -2 g/L; MgSO4.7H2O-0.2 g/L; Na2HPO4– 0.6 g/L; Yeast Extract – 0.2 g/L and trace elements – 1ml was prepared in distilled water. The composition of the trace element solution was FeSO4.7H2O – 10g/L; ZnSO4.7H2O – 2.25 g/L; CuSO4.5H2O – 1 g/L; MnSO4.5H2O – 0.5 g/L; CaCl2.2H2O – 0.2 g/L; Na2B4O7.7H2O – 0.23 g/L; (NH4)8Mo7O24 – 0.1 g/L and 35% HCl 10 mL/L. The N-limited medium was supplemented with 1% of substrates, i.e., sawdust, as carbon source.
2.7. Quantitative determination of the PHB
Fermentation media with the substrate (Tables 3) was inoculated with the select bacteria. After 48 hours of incubation, 10 mL of bacterial culture was centrifuged at 6000 rpm for 15 min. Then, the pellet was suspended in 5 mL of sterile water and dried for 24 h at 100ºC. To the cell suspension, 5 mL of sodium hypochlorite solution was added and incubated at 60ºC for 1 h [20]. This suspension was centrifuged at 6000 rpm for 15 min and the supernatant was separated. To extract cell lipids and other molecules (except PHB) from the supernatant, we added 5 mL of 96% (1:1 v/v) ethanol and acetone. Now, 10 mL of chloroform were added to the tube by placing it in a hot water bath (60°C). Chloroform was evaporated to obtain PHB crystals. 10 mL of 98% H2SO4 was added at 60°C and kept for 1 h to convert PHB crystals into crotonoic acid. After cooling to 25°C, the amount of PHB was determined spectrophotometrically at 235 nm against H2SO4 as a blank with crotonoic acid as a standard [21].
2.8. Optimization of the PHB production
The select bacterial isolate was optimized for different physical conditions to obtain higher PHB crystal yields. The effects of pH (5, 7, 9), temperature (25°C, 37°C, 50°C) and aeration conditions (aeration, non-aeration) on PHB production were recorded. All experiments were conducted in replicates of three. The statistical analysis was performed to find the significant difference among different conditions.
3. Results and Discussion
Bioplastics are biodegradable plastics, produced cheaply by using agro-industrial wastes and as a result are considered non-harmful for our environment. These agro-industrial wastes are converted into simple sugars by bacterial enzymes and used for their energy yields [22].
3.1. Isolation of PHB producing bacteria from industrial soil
In the present project, eight sawdust utilizing bacteria designated as SD1, SD2, SD3, SD4, SD5, SD6, SD7 and SD8 were isolated from sawdust containing medium. Their colonial and physicochemical characterization are presented in the Table 1 and 2.
Table 1: Colonial characterization of the saw dust utilizing bacterial isolates on the Nutrient Agar plates
Isolate Code | Shape | Margin | Elevation | Size | Texture | Appearance | Pigmentation | Optic property |
SD1 | Circular | Entire | Raised | Small | Rough | Dull | White | Opaque |
SD2 | Circular | Entire | Slightly raised | Small | Smooth | Shiny | Off-White | Opaque |
SD3 | Circular | Entire | Raised | Small | Rough | Dull | White | Opaque |
SD4 | Irregular | Undulate | Slightly raised | Small | Smooth | Shiny | Off-White | Opaque |
SD5 | Irregular | Undulate | Flat | Small | Rough | Dull | Off-White | Translucent |
SD6 | Irregular | Undulate | Flat | Small | Rough | Dull | Off-White | Translucent |
SD7 | Irregular | Undulate | Slightly raised | Small | Smooth | Shiny | Off-White | Opaque |
SD8 | Circular | Entire | Raised | Small | Rough | Dull | White | Opaque |
Table 2: Physicochemical properties of saw dust utilizing bacterial isolates
Isolate Code | Shape of the cell | Endospore Staining | Gram Staining | Motility Test | Catalase Test | Oxidase Test | Genus Identified |
SD1 | Rod Shape | Terminal Endospore | Gram Positive | Motile rods | Positive | Negative | Bacillus |
SD2 | Rod shape | Endospore | Gram Positive | Motile rods | Positive | Negative | Bacillus |
SD3 | Diplobacilli | Absent | Gram Negative | Non-motile | Positive | Negative | Klebsiella |
SD4 | Rod Shaped | Terminal Endospore | Gram Positive | Motile rods | Positive | Negative | Bacillus |
SD5 | Diplobacilli | Central Endospore | Gram Positive | Motile rods | Positive | Negative | Bacillus |
SD6 | Diplobacilli | Central Endospore | Gram Positive | Motile rods | Positive | Negative | Bacillus |
SD7 | Rod Shaped | Terminal Endospore | Gram Positive | Motile rods | Positive | Negative | Bacillus |
SD8 | Diplobacilli | Absent | Gram Negative | Non-motile | Positive | Negative | Klebsiella |
3.2. Qualitative Screening of bacterial isolates for bioplastic production
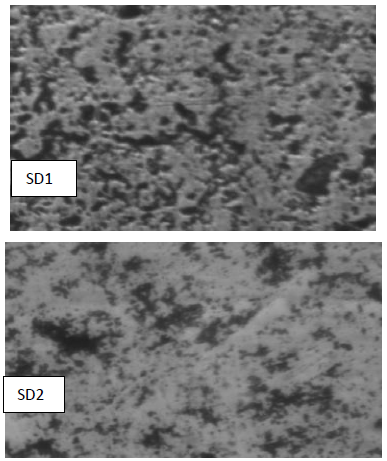
All of the bacterial isolates from the sawdust substrate were initially screened qualitatively by the Sudan Black B staining method, for PHB production. PHB granules were present in the bacterial cells in the form of dark granules. The photomicrographs of isolates showing the PHB granules produced in the form of dark spots in the bacterial cells are given in Fig. 1. Sawdust, being a waste, is affordable and easily available substrate. It contains cellulose, hemicellulose and lignin. Owing to these nutrients, it was used in this study as a substrate to optimize the PHB production. Sawdust is one of the substrates with a higher C/N ratio. The higher carbon and lower nitrogen levels are the major requirements for the higher PHB yields. The nitrogen-limiting condition promotes higher levels of PHB [23], whereas higher nitrogen levels inhibit PHB production by bacteria [24].
Five sawdust utilizing bacterial isolates, SD1, SD2, SD3, SD4 and SD5 showed higher bioplastic granules accumulation. Those showing granules were further assessed for the quantitative production of PHB.
3.3. Screening of the bacterial isolates utilizing sawdust as a low-cost waste for PHB production
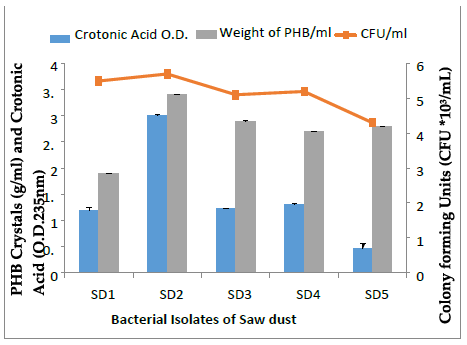
Five distinct bacterial colonies isolated from the industrial soil on the agar plates containing saw dust showed varying levels of CFU/mL, PHB crystal and crotonic acid (Fig. 2-4). Highest CFU/mL was obtained by SD2 (5.7 × 103) which was higher than the SD1 (5.5 × 103), SD3 (5.1 × 103), SD4 (5.2 × 103) and SD5 (4.3 × 103). Maximum PHB (g/mL) crystals production was obtained by SD2 (0.30g ± 0.001/ml) as compared to SD1 (0.19g ± 0.001/ml), SD3 (0.29 g ± 0.001/ml), SD4 (0.27 g ± 0.001/ml) and SD5 (0.28g ± 0.001/ml). SD2 PHB crystals produced higher crotonic acid of 0.31 ± 0.00 optical density at wavelength 235 nm as compared to the SD1 (0.11 ± 0.09), SD3 (0.12 ± 0.15), SD4 (0.13 ± 0.009) and SD5 (0.04 ± 0.09).


3.4. Optimization of the PHB yields by the bacterial isolate SD2
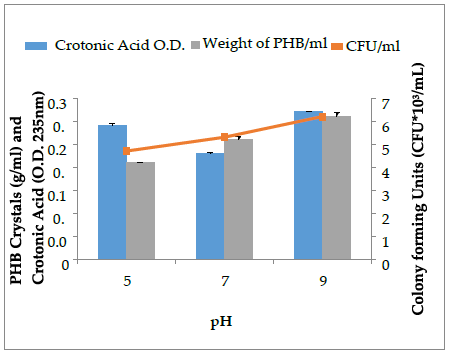
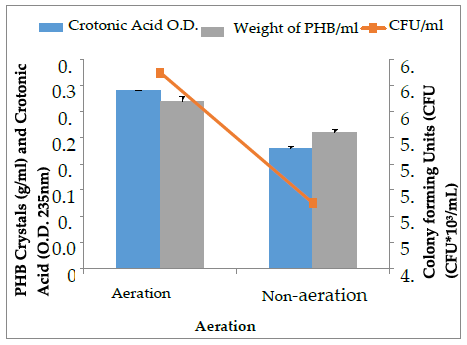
The select bacterial isolates (SD2) with higher PHB crystal accumulation (upto 0.33 g/mL) were optimized for PHB production under varying physical conditions at 48 h of incubation. The effect of pH (5, 7, 9,), temperature (25˚C, 37 ˚C, 50 ˚C) and aeration conditions (aeration, non-aeration) on PHB production by the SD2 isolate was assessed and is presented in Figure 5, 6 and 7. The crystals so produced were further assessed quantitatively by sulphuric acid assay. The pH 9 was found to be optimal for higher PHB and crotonic acid production by the isolate SD2. Many researchers [18] have reported that neutral pH is optimal for maximum PHB production. Oxygenated conditions favor more PHB and crotonic acid production, while the non-aeration conditions lessen the production of PHB. Bacillus species are mostly aerobes that need oxygen to survive. More oxygen concentration favors more bacterial growth and in turn, more PHB is accumulated [25]. It is reported that bacterial cell density and PHB are directly proportional to each other up to a certain level [26].
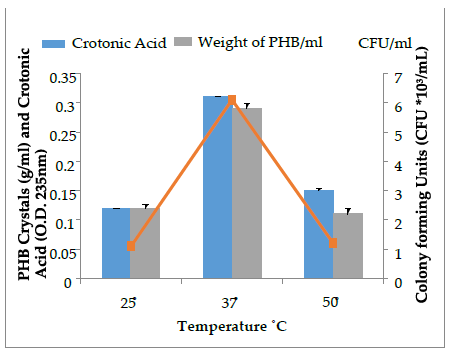
Maximum bacterial growth and PHB production of SD2 (6.3×103 CFU/mL; 0.32 g/mL) was obtained at 37˚C after 48 h of incubation. Bacterial isolates with high growth rate and ideal procedure conditions have been reported for 90% of PHB production [27]. High temperature does not favor increased PHB production as it disrupts the cytoplasmic membrane of the cell. The PHB cannot be stored in the bacterial cell in the presence of high temperature [28]. As temperature reaches its extreme, a sharp decline in the PHB production occurs. Bacterial growth and PHB production are related to each other. When bacterial growth increases, polyhydroxybutyrate also increase to maximum level. After a certain level, PHB production starts decreasing because nutrient depletion occurs and bacteria starts using PHB to obtain the energy [29].
3.5. Identification of the PHB producing SD2 isolate by 16Sr DNA sequencing
The PHB producing SD2 isolate was identified as Bacillus cereus-SD2 through BLAST analyses and was allotted OR607937 accession number on submission to NCBI database. Most of the sawdust utilizing bacterial isolates belonged to the Bacillus genus. Many reporters have described Bacillus species ideal for PHB production [30].
4. Conclusion
In conclusion, our study successfully explored the utilization of sawdust, a significant agricultural waste product, as a substrate for the production of bioplastics. Through a systematic approach, we isolated and optimized bacteria with the ability to produce bioplastics using sawdust as an economical and sustainable substrate. Among the eight indigenous bacterial isolates obtained from an industrial soil sample, Bacillus cereus strain SD2 stood out as the most proficient producer of polyhydroxybutyrate (PHB) when exposed to sawdust. These findings underscore the substantial potential of sawdust as a valuable resource for bioplastic production, thereby addressing the environmental concerns associated with traditional plastics. Moreover, the exceptional performance of Bacillus cereus strain SD2 in bioplastic production, when subjected to optimized conditions, represents a promising avenue for developing eco-friendly alternatives to conventional plastics. For future prospects, further research can focus on scaling up the production process, exploring different strains, and refining the conditions to enhance bioplastic yields. Additionally, investigating the biodegradability and overall environmental impact of these bioplastics will be crucial to fully assess their sustainability and applicability in reducing plastic pollution. Ultimately, our study contributes to the ongoing efforts to transition toward more sustainable and environmentally responsible materials in various industries.
- S. Carmen, “Microbial capability for the degradation of chemical additives present in petroleum-based plastic products: A review on current status and perspectives,” Journal of Hazardous Materials, vol. 402, pp. 123534,2021,doi.org/10.1016/j.jhazmat.2020.123534.
- S.K. Bhatia, S.V. Otari, J.M. Jeon, R. Gurav, Y.K. Choi, R.K. Bhatia, A. Pugazhendhi, V. Kumar, J. RajeshBanu, J.J. Yoon, K.Y. Choi and Y.H. Yang, “Biowaste-to-bioplastic (polyhydroxyalkanoates): Conversion technologies, strategies, challenges, and perspective,” Bioresour. Technol., vol. 326, pp. 124733, 2021,
- I. E. Napper and R.C. Thompson, “Plastic Debris in the Marine Environment: History and Future Challenges,” Global Challenges, vol. 4, no. 3, pp. 1900081, 2020, doi.org/10.1002/gch2.201900081.
- M. A. Burgos-Aceves, H. G. Abo-Al-Ela and C. Faggio, “Physiological and metabolic approach of plastic additive effects: Immune cells responses,” Journal of Hazardous Materials, vol. 404, pp. 124114,2021,doi.org/10.1016/j.jhazmat.2020.124114.
- T. Narancic and K.E. O’Connor, “Plastic waste as a global challenge: are biodegradable plastics the answer to the plastic waste problem?” Microbiology, vol. 165, no. 2, pp. 129–137, 2019, doi.org/10.1099/mic.0.000749 .
- S. A. Kojuri, K. Issazadeh, Z. Heshmatipour, M. Mirpour and S. Zarrabi, “Production of Bioplastic (Polyhydroxybutyrate) with local Bacillus megaterium isolated from petrochemical wastewater,” Iranian Journal of Biotechnology, vol. 19, no. 3, pp. 2849, 2021,doi.org/10.30498/ijb.2021.244756.2849.
- J. Fradinho, L. D. Allegue, M. Ventura, J. A. Melero, M. A. M. Reis and D. Puyol, “Up-scale challenges on biopolymer production from waste streams by purple phototrophic bacteria mixed cultures: A critical review,” Bioresour. Technol. Vol. 327, pp. 124820, 2021,
- J.d.J. Franco-Le´on, E. Arriola-Guevara, L. A. Su´arez-Hern´andez, G. Toriz, G. Guatemala- Morales and R. I. Corona-Gonz´alez, “Influence of supplemented nutrients in tequila vinasses for hydrogen and polyhydroxybutyrate production by photofermentation with Rhodopseudomonas pseudopalustris.” Bioresour. Technol. Vol. 329, pp.124865, 2021,
- R. Sirohi, J. Prakash Pandey, V. Kumar Gaur, E. Gnansounou and R. Sindhu, “Critical overview of biomass feedstocks as sustainable substrates for the production of polyhydroxybutyrate (PHB),” Bioresour. Technol, vol. 311, pp. 123536, 2020,
- R. Z. Sayyed, S. S. Shaikh, S. J. Wani, M. T. Rehman, M. F. Al Ajmi, S. Haque and H. A. El Enshasy, “Production of Biodegradable Polymer from Agro-Wastes in Alcaligenes sp. and Pseudomonas sp.,” Molecules, vol. 26, no. 9, pp. 2443, 2021, doi.org/10.3390/molecules26092443.
- L. Kaur, R. Khajuria, L. Parihar, and G. Dimpal Singh, “Polyhydroxyalkanoates: Biosynthesis to commercial production- A Review,” Journal of Microbiology, Biotechnology and Food Sciences, vol. 6, no. 4, pp. 1098–1106, 2017,doi.org/10.15414/jmbfs.2017.6.4.1098-1106.
- J. Wang, S. Liu, J. Huang, and Z. Qu, “A review on polyhydroxyalkanoate production from agricultural waste Biomass: Development, Advances, circular Approach, and challenges,” Bioresource Technology, vol. 342, pp. 126008,2021,doi.org/10.1016/j.biortech.2021.126008.
- T. M. Keenan, S. W. Tanenbaum, A. J. Stipanovic and J. P. Nakas, “Production and Characterization of Poly-β-hydroxyalkanoate Copolymers from Burkholderia cepacia Utilizing Xylose and Levulinic Acid,” Biotechnol. Prog. Vol. 20, pp. 1697–1704, 2004,
- J. A. Silva, L. M. Tobella, J. Becerra, F. Godoy and M. A. Martínez, “Biosynthesis of poly-β-hydroxyalkanoate by Brevundimonas vesicularis LMG P-23615 and Sphingopyxis macrogoltabida LMG 17324 using acid-hydrolyzed sawdust as carbon source.” J. Biosci. Bioeng. Vol. 103, pp. 542–546, 2007,
- J. A. Posada, J. M. Naranjo, J. A. López, J. C. Higuita and C. A. Cardona, “Design and analysis of poly-3-hydroxybutyrate production processes from crude glycerol,” Process Biochemistry, vol. 46, no. 1, pp. 310–317, 2011,doi.org/10.1016/j.procbio.2010.09.003.
- R. Sindhu, A. Manju, P. Mohan, R. O. Rajesh, A. Madhavan, K. B. Arun, S. H. Hazeena, A. Mohandas, S. P. Rajamani, A. Puthiyamadam, P. Binod and R. Reshmy, “Valorization of food and kitchen waste: An integrated strategy adopted for the production of poly-3-hydroxybutyrate, bioethanol, pectinase and 2, 3-butanediol,” Bioresour. Technol, vol. 310, pp. 123515, 2020,
- H. J. Benson 2020. ‘Microbiological Applications, Laboratory Manual in General Microbiology’’ W.M.C. Brown Publishers, Dobuque, USA
- H, Li, F. Medina, S. B. Vinson, C.J. Coates“Isolation , characterization and molecular identification of bacteria from red imported ant midgut’’ journal of invertebrate pathology, vol. 89, pp. 203-209, 2005.
- R. Sindhu, B. Ammu, P. Binod, S. K. Deepthi, K. B. Ramachandran, C. R. Soccol and A.Pandey, “Production and characterization of poly-3-hydroxybutyrate from crude glycerol by Bacillus sphaericus NII 0838 and improving its thermal properties by blending with other polymers,” Brazilian Archives of Biology and Technology, pp. 54, no. 4, pp. 783–794, 2011, doi.org/10.1590/S1516-89132011000400019.
- S. V. Kumar and S. Ashish, “Production of Polyhydroxybutyrate from Cafeteria Waste: An Environment Friendly Approach,” Pharmacia, vol. 1, no. 2, pp. 47–51. 2011,
- A. Soam, A. K. Singh, R. Singh and S. K. Shahi. “Optimization of culture conditions for bio-polymer producing Bacillus mycoides (WSS2) bacteria from sewage,” International journal of current innovation and research, vol. 1, pp. 27-32, 2012,
- A. Di Bartolo, G. Infurna and N. T. Dintcheva, “A Review of Bioplastics and Their Adoption in the Circular Economy,” Polymers, vol. 13, no. 8, pp. 1229, 2021, doi.org/10.3390/polym13081229
- Lee, Y. R., Fitriana, H. N., ‘’Molecular profiling and optimization studies for growth and PHB production conditions in Rhodobacter sphaeroides’’ Energies, vol.13, no. (23), (2020), doi.org/10.3390/en13236471
- E. Z. Gomaa, “Production of polyhydroxyalkanoates (PHAs) by Bacillus subtilis and Escherichia coli grown on cane molasses fortified with ethanol,” Brazilian Archives of Biology and Technology, vol. 57, no. 1, pp. 145–154. 2014, doi.org/10.1590/S151689132014000100020.
- L. Djerrab, Z. Chekroud, A. Rouabhia, M. A. Dems, I. Attailia, L. I. R. Garcia, and M. A. Smadi, “Potential use of Bacillus paramycoides for the production of the biopolymer polyhydroxybutyrate from leftover carob fruit agro-waste,” AIMS Microbiology, vol. 8, no. 3, pp. 318–337, 2022, doi.org/10.3934/microbiol.2022023
- X. R. Jiang, Z. H. Yao, and G. Q. Chen, “Controlling cell volume for efficient PHB production by Halomonas,” Metabolic Engineering, vol. 44, pp. 30–37. 2017. doi.org/10.1016/j.ymben.2017.09.004.
- J. A. Posada, J. M. Naranjo, J. A. López, J. C. Higuita and C. A. Cardona, “Design and analysis of poly-3-hydroxybutyrate production processes from crude glycerol,” Process Biochemistry, vol. 46, no. 1, pp. 310–317, 2011, doi.org/10.1016/j.procbio.2010.09.003.
- D. Tripathi, A. Yadav, A. Jha and S. K. Srivastava, “Utilizing of Sugar Refinery Waste (Cane Molasses) for Production of Bio-Plastic under Submerged Fermentation Process,” Journal of Polymers and the Environment, vol. 20, no. 2, pp. 446–453, 2012, doi.org/10.1007/s10924-011-0394-1.
- W.N. Chaudhry, N. Jamil, I. Ali, M. H. Ayaz and S. Hasnain, “Screening for polyhydroxyalkanoate (PHA)-producing bacterial strains and comparison of PHA production from various inexpensive carbon sources,” Annals of Microbiology, vol. 61, no. 3, pp. 623– 629, 2011, doi.org/10.1007/s13213-010-0181-6.
- R. Andler, V. Pino, F. Moya, E. Soto, C. Valdés and C. Andreeßen, “Synthesis of poly3-hydroxybutyrate (PHB) by Bacillus cereus using grape residues as sole carbon source,” International Journal of Biobased Plastics, vol. 3, no. 1, pp. 98–111, 2021, doi.org/10.1080/24759651.2021.1882049
No related articles were found.
