Four Rivers and a Reservoir – the Last Homes of the Wild Australian Lungfish
Journal of Engineering Research and Sciences, Volume 3, Issue 1, Page # 11-19, 2024; DOI: 10.55708/js0301003
Keywords: Endangered, Extinction
(This article belongs to the Section Agricultural Engineering (AGE))
Export Citations
Cite
Kemp, A. (2024). Four Rivers and a Reservoir – the Last Homes of the Wild Australian Lungfish. Journal of Engineering Research and Sciences, 3(1), 11–19. https://doi.org/10.55708/js0301003
Anne Kemp. "Four Rivers and a Reservoir – the Last Homes of the Wild Australian Lungfish." Journal of Engineering Research and Sciences 3, no. 1 (January 2024): 11–19. https://doi.org/10.55708/js0301003
A. Kemp, "Four Rivers and a Reservoir – the Last Homes of the Wild Australian Lungfish," Journal of Engineering Research and Sciences, vol. 3, no. 1, pp. 11–19, Jan. 2024, doi: 10.55708/js0301003.
The environment of the Australian lungfish, Neoceratodus forsteri, in south east Queensland, has changed fundamentally since white settlement, and this threatens the survival of the species. Some of the damage to lungfish habitats is the result of human determination to use water for the various needs of industry and people. Additional problems include droughts and floods, as well as loss of plant or animal biodiversity of value to basal fishes like lungfish. Submerged aquatic plants used by lungfish as spawning sites and refuges for the young have been significantly reduced, and food animals for adults and hatchlings are absent or much less common. Without appropriate nutrition for adults, eggs lack the right nutrients for young lungfish. They are unable to develop properly, and die at an early age. Populations of the Australian lungfish in south east Queensland are no longer reproducing sufficiently to guarantee survival of the species in wild habitats of south east Queensland. Lungfish have already died out in Enoggera Reservoir, one of the localities to which lungfish were introduced in 1896. Lungfish will soon be extinct in the four remaining rivers to which they are endemic, because so much biodiversity has been lost. They may survive for a while in the protected environments of zoological parks and aquaria, but not in the habitats where they evolved and lived for so long.
1. Introduction
Lungfish first appeared in the Devonian, and have been found in fossil deposits in many continents and in most epochs since then. Two major families of lungfish are still living, although all of the other lungfish groups are extinct. The Australian lungfish, Neoceratodus forsteri, belongs to the Neoceratodontidae, that first split away from the Lepidosirenidae, with four species in Africa and one in South America, in the early Triassic [1]. The group that includes N. forsteri colonised many environments in central and eastern Australia in the past [2]. Most of these species, from Tertiary deposits in Australia, are now extinct, except for N. forsteri, surviving in coastal rivers that are much affected by water impoundments, in southeast Queensland. This contribution discusses the plight of the last remaining populations of N. forsteri.
The Australian lungfish has lived through many changes to its habitat since it became known to scientists in 1870 [3]. The species has faced droughts and floods, and significant interference from humans, such as habitat loss, reduction in biodiversity in rivers, and the building of water impoundments. Initially, lungfish were considered to be found only in the Mary and Burnett Rivers [4], but recent research has shown that lungfish are endemic to the Brisbane River and possibly also the Pine River as well as the Mary and Burnett Rivers [5]. That N. forsteri is endemic to the Brisbane River has now been accepted by other lungfish researchers [6], although their evidence for this statement is unfortunately based on an incorrect assignment of a fossil lungfish found in a well in the estuary of the Brisbane River [2]. One of the early translocations of lungfish in 1896 placed them in an isolated water impoundment, known as Enoggera Reservoir. They have now died out in this habitat.
Adult lungfish are present, and still numerous, in most environments, although eggs and hatchlings are vulnerable and face many hazards. Apart from accidental catches by fishermen, and human interference with their habitat, the only real threats that may affect adult lungfish in a natural environment are reduced food supplies and old age. Unfortunately, the situation faced by young lungfish is very different. They require refuge from numerous predators in the environment, as well as shelters and food supplies, all now absent from most habitats where lungfish live.
Lungfish are now seriously endangered in all of their wild environments. Recruitment has failed in many habitats. Reasons involve the loss of biodiversity in the rivers and reservoirs, lack of suitable food, and damaged spawning sites. Spawning has ceased in many environments. Rivers are either wrecked by drought or flooding or have been turned into reservoirs. Even if the lungfish manage to spawn, lack of appropriate nutrition for the parents means that eggs and embryos are not properly provisioned and all of the young are affected by deficiencies in the biochemical components of the eggs laid down by the parents [7–9]. The effects of these changes are already catastrophic.
2. Methods
Data on the distribution of lungfish in their current habitats of isolated coastal rivers was derived from literature. Electrofishing to assess the size of the adult population of lungfish was not done, because the main issue facing the species is lack of food and problems with spawning and recruitment. Adult lungfish are still common in parts of the rivers, but young lungfish are absent. Information on the difficulties faced by lungfish in their current environments came from field observations over many years of research, with some published information.
3. Results
3.1. The Burnett River
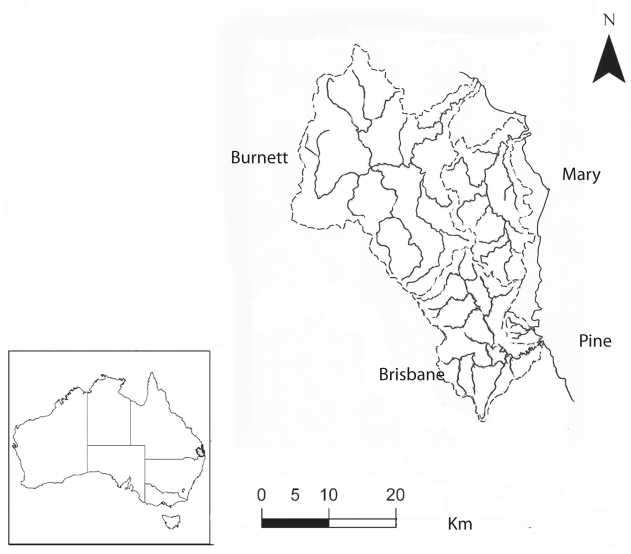
The Burnett River system, the largest of the coastal rivers that are home to lungfish, extends over a wide area of southeast Queensland (Fig. 1). The river rises near Mount Gaeta in the foothills of the Great Dividing Range and joins the sea in Hervey Bay near Fraser Island. Originally surrounded by forests, the Burnett River catchment is now home to numerous sugar cane farms and other agricultural activities.
The river has many tributaries, most now blocked by reservoirs, built since the 1960’s, and some, such as the Ned Churchward Weir and Paradise Dam on the main river, more recently. The most famous of the tributaries is Auburn, the ancestral home of the Australian lungfish [3], now notable for a small national park near a waterfall. The Auburn is well into the hinterland, a long way from the coast. Lungfish are no longer present in this part of the Auburn River, and they are, in effect, extinct in their type locality. The lungfish is now confined to the main river and short regions of major tributaries [10].
Scientists are often the last to take note of new animals, and this is certainly true of the Australian lungfish. The fish were always well known to Aborigines, and later to early settlers. Both of these groups used them for food. There is one record from 1842, describing a story told by a “wild white man” who escaped from a penal settlement in 1829 and lived for many years with a tribe of Aborigines [11]. They often ate lungfish. The escaped convict, Jem Davies, was the first white person ever to see a lungfish.
The first scientific description of the lungfish appeared in a short article by Krefft in 1870 [3], using two preserved adult fish from the Auburn River. Eggs and embryos of the lungfish were not found for another fourteen years, and not described in any detail until a German naturalist, Richard Semon, came to the McCord property in Queensland in 1892 to collect and study the eggs and take numerous examples home to be examined by his colleagues in Europe [12]. Semon has the distinction of finding the first wild hatchling, one inch long, among submerged water plants in the river, the only wild hatchling to be discovered for many years. This gave rise to the frequently repeated comment that juvenile lungfish were rarely to be found, and the species was on the brink of extinction. For many years, this statement proved to be needlessly pessimistic. Although few wild juveniles have been recorded, lungfish have survived. Now, it could well be prophetic. Lungfish are in real trouble, because food supplies for adults and young are now reduced. A balanced system of long-lived adult fish, and low recruitment, operating for millennia, has been thrown out of equilibrium.
The Burnett River originally flowed more or less as nature intended, with numerous tributaries that united to form meandering rivers, having many long, deep pools and short stretches of rapid flow. The river was affected by floods, and in times of drought was reduced to “chains of waterholes”, but fish and other animals retreated to the deep pools left behind, and colonized the river when it began to flow again. The water holes were extensive, and retained populations of plants and animals, including lungfish, throughout the drought. The river was affected by agricultural activities, and by towns and villages, and the occasional disaster when unwanted effluent from factories entered the river, but there were no major interruptions to the flow of the river. Plenty of habitats remained for the lungfish, throughout the whole system.
The early Burnett system is a far cry from the debased and altered river that exists now. The Burnett River now has 26 water impoundments, and many are impassable to lungfish. There are very few fishways on any of the water impoundments, and lungfish often ignore them when they are present [13, 14].
Caldwell was the first to find spawn of the lungfish, in a number of different sites in the Burnett River, assisted by Indigenous people, and the first to attempt to raise the eggs and embryos [15]. Caldwell does not list the numbers of viable versus dead eggs. In [16], the author was the first to raise lungfish to young juveniles, well beyond the tricky stages of egg and hatchling development, but he published little of his methods. He found eggs on Vallisneria plants. In [17], the author worked for many years, collecting eggs and attempting to raise them, often with little success because he failed to recognise that young lungfish are not filter feeders, and, until they reach a certain size, are incapable of breathing air because the lungs have not yet developed. He was much less successful than Illidge. In [17], the author considered that the weeds on which lungfish spawn are Vallisneria, Hydrilla and, if nothing else is there, Nitella. Espinoza and his colleagues [18] made collections of eggs but give little information on development and recruitment, or even how many eggs were actually healthy and viable.
Later studies by State Government officers have listed an enormous number of weeds on which eggs were found, and some records of juveniles and subadults [10]. Weeds used for spawning were species of Vallisneria, Hydrilla, Nitella and Potamogeton, as well as Myriophyllum, and even grass. They refer to the proportion of living eggs in the field, higher as macrophyte density increased. The number of macrophytes apparently used in certain years reflects eggs being shed loose into the water current, caught by any weed in the current and not specifically laid there during spawning. In those years when spawning was prolific, eggs were also found in rocky pools in the river, bereft of any plant cover. The work of these authors is now twenty years old and no further information has appeared [10].
3.2. Mary River
The Mary River, south of the Burnett River, rises at Booroobin, west of the town of Landsborough, and flows north to the Great Sandy Strait on the coast of Queensland (Fig. 1). Historically, the Mary River was a perfect environment for lungfish, with deep pools, plenty of submerged aquatic vegetation, fish and invertebrates, rainforests growing on the banks, and short stretches of shallow fast flowing water [19].
Gradually, the surrounding land was taken for farms, causing some changes, but the river was not otherwise much altered in historical times, except perhaps by cattle grazing on the banks. The Mary River was the source of four adult lungfish preserved and sent to London in 1870, where they formed the basis of a description by Gunther [4] in the following year.
The Mary River already has 11 major water impoundments, and the plan to turn the whole river into a long, shallow water impoundment, to be known as Traveston Dam, was rejected by the Federal Government. However, several tributaries of the Mary River have been turned into reservoirs, such as Borumba Dam over Yabba Creek, built in 1964. These water impoundments help to supply water to the Mary Valley residents, and support irrigation for farming.
A single collection of lungfish eggs and embryos was made in Imbil late in the season of 1976 [20]. Eggs were found among water plants in two or more feet of water. No data on the number of viable eggs was recorded. There are few additional records of spawning and egg collections in the Mary River, but, at times, large numbers of juvenile lungfish have been collected and measured [10]. Little information is available on survival of eggs and embryos from the Mary River.
3.3. Brisbane River
South of the Mary River, and separated from it by the mountains of the Conondale Ranges, is the Brisbane River system (Fig. 1). The Brisbane River rises in the foothills of the Great Dividing Range east of the town of Kingaroy, and joins the Stanley River, the largest tributary, which originates in the Conondale ranges. The Brisbane River is a large permanent system with many tributaries, some small and insignificant and others extensive, meandering across a wide area of mostly flat ground, starting in the mountains and discharging to the sea on the east coast. The Brisbane River is similar to the Mary River in fauna and flora, and in water composition, but much deeper and flowing faster in the unaltered reaches.
The early descriptions of the author are lyrical, presenting an idyllic picture [21]. After white settlers began to alter the river, for farming or habitation, this changed rapidly. The estuary of the river is affected by tidal flows, and by dredging, initially to allow for navigation and later, in 1862, to provide gravel for the building trade. This has made the river, for much of its length, murky and not particularly salubrious. Although dredging ceased many years ago, and navigation by large ships is now confined to the Port of Brisbane, the lower Brisbane River is still muddy. This does not apply to the upper reaches of the river, where the water was, and still is, clean and fresh.
Prior to 1895, when it was decided to move lungfish around the state, to ensure the survival of the species, there are no written records of lungfish in the Brisbane River. However, lungfish bones 3500 years old were found in an Aboriginal site, the Platypus Rockshelter, near Northbrook on the Brisbane River, a long way from the Mary River and from the Burnett River [5]. Lungfish are endemic in the Brisbane River.
Much of the Brisbane River flowed through open country with sparse eucalypt forest, often burnt by Aborigines to encourage new growth and attract herbivorous prey animals. Riparian vegetation at that time included many rainforest elements. Bottlebrush trees growing close to the river bank provided masses of fine rootlets suspended in the water where lungfish could lay their eggs and hatchlings could find food and hiding places. The entire system of riparian vegetation provided a shady and sheltered environment. This has now changed, and the Brisbane River catchment is affected by two large reservoirs, Lake Wivenhoe and Lake Somerset, with no fishways, taking up much of the original river. The oldest reservoir, Mount Crosby Weir, is small, and does have a fishway, which lungfish are too heavy to use.
The Brisbane River was originally a rich environment for aquatic animals. There were extensive beds of eelgrass in shallows and deep water at Northbrook, and at Fernvale, just below Twin Bridges, where small clams such as Corbicula lived. At Lowood a few kilometres upstream of Twin Bridges, there were snails such as Thiara living on rocks and among weeds as well as Corbicula in the shallows. In all areas, there were banks of water plants in deep water, as well as river bottlebrush growing along the edge of the river, with roots hanging into the water, home of Corbicula, rotifers, worms, small prawns, sponges, little fish, and a favourite site for lungfish to spawn [22]. This picture was repeated along the length of the river, outside the towns and villages.
Before Lake Wivenhoe was built, lungfish spawned in many sites along the river, mostly on the submerged rootlets of Callistemon trees growing on the river bank, or on the roots of Lomandra growing close to the Callistemon trees. In shallow areas, they laid eggs on the leaves of extensive beds of Vallisneria, and occasionally in banks of Hydrilla growing in deeper water. The most extensive sites were at Northbrook, close to the Platypus Rockshelter. The season in the River was long, beginning in in the middle of August and continuing until December. Mortalities of eggs collected from these sites were usually low, and most of the eggs collected were newly laid or only a few days old [22]. Food and refuges for hatchling lungfish were plentiful, among the tree rootlets or the Vallisneria plants. This safe and rich environment was destroyed for ever when Lake Wivenhoe was created, and decent spawning sites were only to be found in parts of the river remote from the reservoir. In places like Lowood and Fernvale, lungfish made use of the trailing roots of Callistemon trees, and sometimes of Lomandra plants on the river bank. They also used Vallisneria plants in the shallow water. However, in the last few years, water plants that serve as attachments sites for eggs, and refuges for hatchling fish, have been destroyed in the Brisbane River by flooding, first in 2011 and more recently in 2022. With no water plants, food for hatchlings is gone as well. To make matters worse, food for adult lungfish has mostly disappeared.
Mortalities among the eggs collected from the river below Lake Wivenhoe began to rise after 1992. In some years the lungfish did not spawn, and after 1994, many eggs were shed loose into the current, and attachment to a suitable plant was a matter of chance [22]. Spawning in a second site at Fernvale was even more erratic, and results from this site were highly variable. Spawning ceased in Lowood in 2003, and in Fernvale in 2006.
Some years after spawning stopped in the river environment, eggs were found in Lake Wivenhoe, in a poor environment. Cattle are allowed to graze on the shores of Lake Wivenhoe, and the waters close to the shore are contaminated with cattle faeces. The substrate in the shallows is pitted by deep troughs formed when the cattle walk into the water. Fluctuating water levels leave dead grass in the shallows and there is little Vallisneria. Filamentous algae proliferate in the shallow water.
Spawning in Lake Wivenhoe was haphazard, and none of the eggs were attached to water plants. They were shed loose into shallow water, and were subject to the effects of storms and water movements induced by wind. Some of the eggs drifted into the masses of filamentous algae or into clumps of dead grass. Eggs were often blown towards the bank of the reservoir, into shallow water and exposed to the sun, or caught among dead grass, which does not provide a suitable habitat for development [7].
In [23], the author collected eggs from many sites around the reservoir in 2009, but failed to recognise that most of these eggs were trapped in a poor environment and could not develop. One juvenile, of stage 46, was collected in the reservoir at this time [7]. Under normal circumstances, this juvenile would have started to feed and grow. However, when taken in to the laboratory and placed in a tank with numerous black worms, usually a favourite food, the juvenile failed to show any interest in the food, or to ingest any worms. After a few days, it died, as did all of the other hatchlings from this site [7]. The prolific spawning of 2009 around the shores of Lake Wivenhoe was hailed as a good news story, because it was thought that that lungfish could survive, spawn and do well in a reservoir environment. This is not the case. Few if any of the hatchlings survived, and the spawning of 2009 was not repeated in 2010.
3.4. Pine River
The Pine River system, with two branches, imaginatively labelled South and North, is a coastal river system (Fig. 1), originally similar to the Brisbane River nearby in flora and fauna. The South Pine River is a creek, quite small. Both rivers rise in the foothills of the D’Aguillar ranges, well separated from the Brisbane River catchment, and flow east into the sea at Bramble Bay.
The North Pine River has been virtually obliterated by a single large reservoir, completed in 1976 and known as Lake Samsonvale. Little of the original river remains above the lake, and a short creek extends from the reservoir wall to a weir that separates brackish water from fresh. Most of the lungfish population live in the reservoir, which has no fishway, or in the depauperate creek below the wall, where food is limited, and sites for spawning are absent.
There are no written historical records of lungfish in the Pine River catchment before the translocation experiments in [24]. This does not mean that lungfish were not present in the system. It means that they had not been found there. At least one unique mitochondrial haplotype has been found in the Pine River (Loh, pers.com.), suggesting that the lungfish are endemic in this catchment.
Lungfish spawned in Lake Samsonvale for several years before and after the floods of 2011 [8,9,22]. The season in the lake was always late in the spring, and short. Eggs were invariably laid in shallow water close to the banks, and never attached. Both Vallisneria and Nitella plants were available, but the eggs were always loose. Numbers of dead eggs were higher than in the Brisbane River at Lowood, and eggs were often blown onto the shore if there was a storm, where they died of dehydration and exposure to the sun. A few hatchlings were found among the water plants, all with anomalies, and none survived for long despite reaching the point of hatching in a natural environment. In the last year that eggs were collected, only one female spawned, producing small dark eggs, all of which died.
3.5. Enoggera Reservoir
Enoggera Reservoir is isolated by position and by distance from any river containing lungfish, and certainly had no native population of this species. Despite records of plants and animals that occur in the reservoir, Enoggera is not a rich environment, and the species diversity of plants and animals living in the lake may well have been improved by the introduction of water hyacinth to the reservoir in 1867, because so many small animals can live amongst the roots trailing in the water. When lungfish were placed in the reservoir in 1896 [24], they used the water hyacinth for spawning, and searched for food among the submerged roots.
Enoggera Reservoir has a long shore line, and waterlilies and water hyacinth grew around most of the reservoir, except along the wall of the dam and in the creek that enters the reservoir. Lilies and hyacinth did not become established in either of these places. Despite the huge number of potential sites for spawning, lungfish patronised only three, two in relatively stagnant water and one where the water flowed slowly past the hyacinth. If they used other sites, and eggs did not become fixed to hyacinth roots, eggs would have fallen into deep water and never been found, or be able to develop in the anoxic environment of the deeper levels of the reservoir.
The season of egg laying in Enoggera Reservoir was short, and most of the eggs collected were able to develop and hatch [22]. Several young hatchlings were found among the rootlets of the water hyacinth, close to the egg cases they had recently vacated. On one occasion, a hatchling living among the rootlets was seen slipping back into the egg case, which was still attached to the roots. Food items for hatchling lungfish could be found among the hyacinth roots, and these provided a safe environment of young fish. Air breathing was observed in laboratory reared juveniles, but not until the fish was over an inch long and capable of rising to the surface of the water. Hatchlings have not yet developed a lung and cannot breathe air.
This protected environment for young fish was lost early in 1974. Enoggera Reservoir, at that time retained by the original low earth dam wall, contributed to the flooding of Brisbane suburbs, caused by exceptionally heavy rain. Water hyacinth was carried out of the reservoir and into the creek, along with many fish. After the flood, the water hyacinth was poisoned with herbicides, thus destroying refuges for eggs and young hatchlings. With no protected spawning habitat, no embryos and young lungfish survived. Any adult lungfish collected from Enoggera Reservoir after the hyacinth was cleared were exceptionally old fish, with numerous disease conditions and seriously damaged tooth plates [25]. A few have apparently survived in Enoggera Creek, but this is a poor environment for large fish, because it has little food and no spawning sites.
Clearing of the hyacinth in 1974 was not the first time that this plant had been removed from the reservoir. A few years after in [26] had collected juvenile lungfish, hiding in water hyacinth roots, from Enoggera Reservoir, the weeds were cleared by forking the hyacinth plants onto the bank. At the time, people asked for hyacinth to be allowed to grow back in the reservoir, to protect small animals, and this happened, probably because Nature took a hand in the matter. The hyacinth may not have been completely cleared, and was able to regenerate. Clearing of the hyacinth was a lot more efficient in 1974, using weed killers.
Reasons for the failure of the lungfish of Enoggera Reservoir to recruit any young to the adult population after the water hyacinth was removed from the reservoir were not only a result of the age of the adults, at least immediately after the clearing of the weeds. Without the weed cover, the steep banks of Enoggera Reservoir, when it was denuded of water hyacinth, meant that any eggs laid simply rolled down into deep water that was anoxic and had no refuges. Lungfish continued to carry out their spawning behaviour for several years in Enoggera Reservoir, but the eggs and young had no shelter. The lungfish of Enoggera Reservoir are now extinct [22].
3.6. Diets of lungfish
Juveniles are essentially carnivorous, capturing small animals such as crustacea, insect larvae and small worms with sharp teeth that encircle the mouth [27]. As the dentition gradually morphs into crushing and grinding tooth plates, the diet changes. Adults are suctorial feeders, and ingest masses of sand, detritus and filamentous algae, as well as other water plants and small molluscs such as Corbicula and Thiara, along with occasional small fish and crustacea [28]. Plant material passes through the intestine undigested, along with the sand and detritus, and the adult fish gains most of its nutrition from the molluscs, including the plant material that the molluscs have eaten and digested. In this way, lungfish are able to ingest volatile fatty acids from the plants eaten by the molluscs. Lungfish cannot synthesise volatile fatty acids, and have to obtain them from the diet [7]. Volatile fatty acids are essential for development of the embryos and hatchlings [7–9, 22].
3.7. Lungfish collected from 1990 to now
A number of adult lungfish were collected from Lowood before major changes from flooding and drought had altered the environment and caused problems for lungfish [29]. The intestines, and faeces produced, contained some undigested plant material, and broken fragments of Thiara and Corbicula shells, indicating that the fish were obtaining their nutrients mostly from small molluscs. Wear on the tooth plates was light and showed normal sub-terminal rotational grinding. These fish were feeding well and the population was in good condition.
Adults from Enoggera Reservoir collected at a similar time presented a different picture. The intestines contained a few leaves from Hydrilla, and some broken shells of a small freshwater snail as well as some crustacean carapaces. The diet was not particularly rich or varied. Tooth plates were heavily worn, deeply incised to the mediolingual face, and with numerous carious lesions [29]. Enoggera fish were all old, and the population was no longer actively spawning, since the water hyacinth on which eggs were normally laid had been removed from the reservoir.
Lungfish collected from Lake Samsonvale after the flood of 2011 were in poor condition, and the intestines of most fish were empty. A few contained undigested filamentous algae. Tooth plates were worn smooth, showing attrition and indicating that the fish had not been chewing or grinding food. Fish collected from the headwaters of Lake Wivenhoe at the same time appeared to be in good condition but had empty intestines and tooth plates showing attrition [29]. This is what is happening to surviving populations of lungfish in reservoirs now. Food in river environments is also limited because flooding washes food animals away.
3.8. Spawning and Recruitment
Spawning is initiated by rising photoperiod [30]. It has nothing to do with the availability of water plants or rootlets, with oxygen levels, with rainfall, or water quality, with flowing or stagnant water, or with phases of the moon. Lungfish begin to spawn in early spring, almost always before any significant rain falls. Oxygen levels vary little in most places where lungfish spawn, as does water quality. In a river, submerged water plants or rootlets line the shallow water along the banks, and in a water impoundment, there is usually nothing but dead leaves and filamentous algae, and in one well known instance, cattle faeces and dead grass [23]. Spawning may start and continue in all of these places. Water may be flowing or stagnant, and the only movement induced in the shallows of a reservoir is drift caused by wind.
Under normal circumstances, in a river or lake with stagnant or slowly flowing water and submerged water plants or rootlets, lungfish will carry out a coordinated spawning activity with the pair of fish entwined, the female producing eggs one by one and the male spraying milt as the eggs are laid [30]. Under these circumstances most of the eggs will be fertilised and the percentage of dead and dying eggs on collection will be low. Most of the eggs will be attached to plants or rootlets, and only a few will be shed into the water column. If the fish are attempting to spawn in a water impoundment without adequate submerged water plants or dense masses of rootlets, eggs will be shed loose into the water column, and a much higher percentage of eggs will die early. They will fall onto the substrate or into masses of detritus or filamentous algae. In shallow water, unattached eggs can be affected by water currents induced by wind action on the surface. They can be exposed to heat in the shallows or even washed onto the shore where they will die of exposure. In some water impoundments, where the fish have spawned among patches of Vallisneria plants, eggs may be caught amongst the leaves and afforded some protection, even if they are not attached. Refuges are vital for hiding the eggs and as places where young lungfish can find food. Suitable shelters have been found in water impoundments, such as water hyacinth before it was removed from Enogerra Reservoir, but this is not always the case. If the water level fell, the floating plants of hyacinth were able to follow the levels. With few or no water hyacinth plants, reservoirs have few places where young lungfish can hide.
Periodic floods and droughts in a natural river system have profound effects on spawning and recruitment. In a flood, unprotected eggs can be carried away and lost in deep water. During the long drought of 2001-2008, parts of the Brisbane River were allowed to dry out, and flow was only maintained in the deep channels. Lungfish spawn in the shallows, where Vallisneria grows in patches, and Callistemon rootlets extend into the water. This is also where snails and clams proliferate. The shallows were left dry, so lungfish had no food and no spawning sites, and after some years, spawning ceased [8,9]. In parts of the river where spawning sites were maintained, as in deep water below Callistemon trees, spawning also failed, because there was no food for the adult fish elsewhere in the River. All of these areas were damaged during the flood of 2011, and the damage continued because of the prophylactic water releases to ensure that water impoundments upstream of the spawning sites did not become overfilled. The sites on the Brisbane River have not yet recovered their vegetation cover completely, after the flood, and lungfish no longer spawn in these River sites. A subsequent flood in 2022 further damaged the river, and the former spawning sites.
In their analysis of the current state of the rivers in Queensland, and discussion of the lungfish populations, Brooks and his colleagues [6] do not mention that large numbers of lungfish hatchlings die before thay can complete development and be recruited to the adult population [7–9], and that spawning has ceased in many environments [22]. They state that “rivers are fragmented” but do not point out that there is little food for adults or young lungfish, and that the rivers are so damaged by floods and droughts that shelters for young lungfish are now absent.
4. Discussion
Aquatic animals, especially hatchlings and young juveniles, cannot be separated from their environment, and are completely dependent on water, refuges and food within that environment. They cannot escape from floods and droughts, or from human changes imposed on the rivers where they live, and habitat degradation affects both adults and young.
Electrofishing to determine the number of adults still living in the environments of the lungfish was not performed during this research. Adults are still present in reasonable numbers in the rivers and reservoirs [6], although during tests to determine ages of lungfish by DNA methylation, the smallest specimens in the study had to be derived from captive reared juveniles [31]. The issue is spawning, recruitment, and the amount of food present for all stages of the life cycle. Electrofishing does not provide any information on these topics, and can be lethal to adult lungfish. Every single one of the adult lungfish provided for my research since 2010 had empty intestines and tooth plates worn flat by attrition, indicating a lack of food [29]. These fish died by accident after flooding.
Changes to the riverine environments of southeast Queensland may have been slow in the beginning, but floods, droughts and water impoundments have accelerated damage to the habitat, and it is now completely depauperate. Fundamental changes to the plants and animals that lived in the rivers and provided the lungfish with shelter and food, and the hatchlings with refuges, have resulted in a habitat that can no longer support the presence of young lungfish. The changes mean that suitable water plants to serve as attachments sites for eggs, and refuges for hatchling fish, have been destroyed in the habitats of the lungfish. The water impoundments have fluctuating water levels, so plants cannot become established, and the small molluscs like Thiara and Corbicula that adult lungfish use for food cannot survive.
Lack of appropriate food is universal in water impoundments, and now in the rivers as well. The most likely cause of poor recruitment is an inadequate diet for the adult fish. Because small molluscs are missing from most water impoundments, and now from the rivers, and because lungfish derive volatile fatty acids from these molluscs in their diet, fish in water impoundments do not get enough volatile fatty acids, especially at the right time, and cannot form eggs with adequate nutrients for development. The epidermis, and sense organs associated with the epidermis, are deficient. So development, and recruitment, fail. Loss of recruitment of new juveniles is a consequence of an inadequate diet for the adults, which should include snails and clams. Adult lungfish cannot digest plant material, so do not obtain volatile fatty acids from their diet unless they eat small molluscs that have been able to feed on plants and digest them. If the adults do not eat enough animal material containing digested plants, the eggs do not contain adequate volatile fatty acids.
Lack of volatile fatty acids in the eggs results in poor development of important epidermal structures, particularly in the skin sense organs and in the ciliated cells on the skin. Hatchlings are unable to find their food, sense danger in the environment, or even to keep their skins clean. They cannot eat food even if they find it, they are unable to digest it, and they cannot defaecate. They are vulnerable to pathogenic bacteria and fungi, and, once the yolk supply is used up, they die. In the Lakes, the anomalies are so common, and so serious, as to destroy all the young of one season. Analysis of the few eggs and embryos collected during the long drought of 2001-2008 has shown that problems with the development of skins and skin sense organs began during this time, and for reasons related to lack of food for the parent fish. In River spawning grounds, loss of suitable weeds, and damage to the river banks, as well as lack of appropriate food for hatchling lungfish and adults, has brought spawning to an end in many places [22]. It could be that, in many parts of the current home range of the lungfish, recruitment of juveniles to the adult population, always low, has been reduced to zero.
5. Conclusions
- Isolated coastal rivers in south east Queensland where lungfish are endemic are now seriously damaged by the building of reservoirs with no fishways, and by droughts and flooding.
- Food for lungfish is now scarce, and shelters for eggs and hatchlings have been destroyed by floods.
- Spawning has ceased in many environments inhabited by lungfish.
- Embryos and hatchling lungfish are no longer viable because adults are affected by lack of appropriate food, and any eggs laid are deficient in appropriate nutrients. Skin structures like sense organs and ciliated skin cells do not develop. Hatchlings cannot sense food, and cannot keep the skin clean.
- Eggs and embryos all die before they complete development.
- Wild lungfish are now threatened with extinction, as admitted by Brooks and colleagues [6].
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgment
Living lungfish described in this paper were collected with permission from the University of Queensland Animal Ethics Committee, approval number CMM/013/03/ARC, and the Queensland Fisheries Management Authority, permit number PRM03012K.
- L. CAVIN, V. SUTEETHORN, E. BUFFETAUT, H. TONG, “A new Thai Mesozoic lungfish (Sarcopterygii, Dipnoi) with an insight into post-Palaeozoic dipnoan evolution,” Zoological Journal of the Linnean Society, vol. 149, no. 2, pp. 141–177, 2007, doi:10.1111/j.1096-3642.2007.00238.x.
- A. Kemp, “Lungfish and the Long Defeat,” Diversity, vol. 15, no. 1, pp. 63, 2023, doi:10.3390/d15010063.
- G. Krefft, Description of a gigantic amphibian allied to the genus Lepidosiren, from the Wide-Bay District, Queensland, The Society, 1870.
- Albert Charles Lewis Gotthilf Gunther, “Description of ceratodus, a genus of ganoid fishes, recently discovered in rivers of Queensland, Australia,” Philosophical Transactions of the Royal Society of London, vol. 161, , pp. 511–571, 1871, doi:10.1098/rstl.1871.0020.
- A. Kemp, L. Huynen, “Occurrence of lungfish in the Brisbane River, Queensland, Australia dates back to 3850 yr BP,” Journal of Archaeological Science, vol. 52, , pp. 184–188, 2014, doi:10.1016/j.jas.2014.08.021.
- I.R. List, “The IUCN red list of threatened species,” Di Sponí Vel Em:< Http://Www. Iucn Red List. Org/Info/Cat e Go Ries_cri Te Ria2001. Html>. Aces so Em, vol. 12, , 2004.
- A. Kemp, “Comparison of embryological development in the threatened Australian lungfish Neoceratodus forsteri from two sites in a Queensland river system,” Endangered Species Research, vol. 15, no. 2, pp. 87–101, 2011, doi:10.3354/esr00358.
- A. Kemp, “Abnormal development in embryos and hatchlings of the Australian lungfish, Neoceratodus forsteri, from two reservoirs in south-east Queensland,” Australian Journal of Zoology, vol. 62, no. 1, pp. 63, 2014, doi:10.1071/ZO13038.
- A. Kemp, “Histological analysis of hatchlings of the Australian lungfish, Neoceratodus forsteri, from water impoundments reveals fundamental flaws in development,” Pacific Conservation Biology, vol. 23, no. 2, pp. 163, 2017, doi:10.1071/PC16036.
- S.G. Brooks, P.K. Kind, Ecology and Demography of the Queensland Lungfish (Neoceratodus Fosteri) in the Burnett River, Queensland with Reference to the Impacts of Walla Weir and Future Water Infrastructure Development, Department of Primary Industries, Queensland Agency for Food and Fibre Sciences, 2002.
- P.J. Tynan, Duramboi: The Story of Jem Davis of Glasgow (1808?-1889), Church Archivists’ Press, 1997.
- Richard Semon, In the Australian Bush, and on the Coast of the Coral Sea: being the Experiences and Observations of a Naturalist in Australia, New Guinea, and the Moluccas, 1899, doi:10.1038/060169a0.
- A.P. Berghuis, C.D. Broadfoot, “Upstream passage of Queensland lungfish at Ned Churchward Weir fishlock,” 2004.
- A.P. Berghuis, C.D. Broadfoot, “Downstream passage of fish at Ned Churchward weir,” 2004.
- W. H. Caldwell, “The eggs and larva of Ceratodus,” Proceedings of the Royal Society of New South Wales, vol. 1, , pp. 138, 1895.
- T. Illidge, “Ceratodus,” Brisbane Courier, 1902.
- T. L. Bancroft, “On the life history of Ceratodus,” Proceedings of the Linnean Society of New South Wales, pp. 315–317, 1928.
- T. Espinoza, S.M. Marshall, A.J. McDougall, “Spawning of the endangered Australian lungfish (Neoceratodus forsteri) in a heavily regulated river: A pulse for life.,” River Research and Applications, vol. 29, no. 10, pp. 1215–1225, 2013, doi:10.1002/rra.2607.
- D.P. Johnson, State of the Rivers: Mary River and Major Tributaries: an Ecological and Physical Assessment of the Conditions of Streams in the Mary River Catchment, Department of Natural Resources, Resource Sciences Centre, 1997.
- H.P. WHITING, Q. BONE, “Ciliary cells in the epidermis of the larval Australian dipnoan, Neoceratodus,” Zoological Journal of the Linnean Society, vol. 68, no. 2, pp. 125–137, 1980, doi:10.1111/j.1096-3642.1980.tb01922.x.
- P. Davie, E. Stock, D.C.L. Choy, A.L. Society, Q. Museum, The Brisbane River: A Source-book for the Future, Australian Littoral Society, 1990.
- A. Kemp, “Changes in the freshwater environments of the Australian lungfish, ‘Neoceratodus forsteri’, in south-East Queensland, and implications for the survival of the species,” The Proceedings of the Royal Society of Queensland, vol. 124, , pp. 121–135, 2020.
- D.T. Roberts, S. Mallett, N.C. Krück, W. Loh, I. Tibbetts, “Spawning activity of the Australian lungfish Neoceratodus forsteri in an impoundment,” Journal of Fish Biology, vol. 84, no. 1, pp. 163–177, 2014, doi:10.1111/jfb.12264.
- D. O’Connor, “On the redistribution of Ceratodus,” Proceedings of the Royal Society of Queensland, vol. 11, , 1897.
- A. Kemp, “New insights into ancient environments using dental characters in Australian Cenozoic lungfish,” Alcheringa: An Australasian Journal of Palaeontology, vol. 29, no. 1, pp. 123–149, 2005, doi:10.1080/03115510508619564.
- H. Longman, “Discovery of juvenile lungfishes with notes on Epiceratodus.,” Memoirs of the Queensland Museum, vol. 9, , pp. 161–173, 1928.
- A. Kemp, “Marginal tooth‐bearing bones in the lower jaw of the recent australian lungfish, Neoceratodus forsteri (Osteichthyes, Dipnoi),” Journal of Morphology, vol. 225, no. 3, pp. 345–355, 1995, doi:10.1002/jmor.1052250306.
- A. Kemp, “The biology of the australian lungfish, Neoceratodus forsteri,” Journal of Morphology, vol. 190, no. S1, pp. 181–198, 1986, doi:10.1002/jmor.1051900413.
- A. Kemp, “Environmental alterations in southeast Queensland endanger the Australian Lungfish, Neoceratodus Forsteri (Osteichthyes: Dipnoi),” The Proceedings of the Royal Society of Queensland, vol. 122, , pp. 45–57, 2017.
- A. Kemp, “Spawning of the Australian lungfish, Neoceratodus forsteri(Krefft) in the Brisbane River and in Enoggera Reservoir, Queensland.,” Memoirs of the Queensland Museum. Brisbane, vol. 21, no. 2, pp. 391–399, 1984.
- B. Mayne, T. Espinoza, D. Roberts, G.L. Butler, S. Brooks, D. Korbie, S. Jarman, “Nonlethal age estimation of three threatened fish species using DNA methylation: Australian lungfish, Murray cod and Mary River cod,” Molecular Ecology Resources, vol. 21, no. 7, pp. 2324–2332, 2021, doi:10.1111/1755-0998.13440.
No related articles were found.

