Jaw and Tongue Muscles in the Australian Lungfish, <i>Neoceratodus forsteri </i> (Osteichthyes: Dipnoi)
Journal of Engineering Research and Sciences, Volume 3, Issue 2, Page # 15-21, 2024; DOI: 10.55708/js0302003
Keywords: Jaw Muscles, Tongue (hyoid) Muscles, Australian Lungfish
(This article belongs to the Section Agriculture Dairy and Animal Science (ADA))
Export Citations
Cite
Kemp, A. (2024). Jaw and Tongue Muscles in the Australian Lungfish, <i>Neoceratodus forsteri </i> (Osteichthyes: Dipnoi). Journal of Engineering Research and Sciences, 3(2), 15–21. https://doi.org/10.55708/js0302003
Anne Kemp. "Jaw and Tongue Muscles in the Australian Lungfish, <i>Neoceratodus forsteri </i> (Osteichthyes: Dipnoi)." Journal of Engineering Research and Sciences 3, no. 2 (February 2024): 15–21. https://doi.org/10.55708/js0302003
A. Kemp, "Jaw and Tongue Muscles in the Australian Lungfish, <i>Neoceratodus forsteri </i> (Osteichthyes: Dipnoi)," Journal of Engineering Research and Sciences, vol. 3, no. 2, pp. 15–21, Feb. 2024, doi: 10.55708/js0302003.
The Australian lungfish, Neoceratodus forsteri, has several muscles associated with feeding. The massive adductor mandibularis muscle of N. forsteri, which closes the jaws, originates on the chondrocranium and inserts on the posterior aspect of Meckel’s cartilage in the mandible. The depressor mandibulae muscle, which opens the jaws, inserts on the medial articulation of the prearticular bones of the mandible and originates on the pectoral girdle. Oblique muscles, originating on the prearticular bone and inserted into tissues of the midline of the mandible, carry out the subterminal grinding movements of the mandible to masticate food. Separate muscles control the hyoid apparatus. Interhyoideus muscles originate on the posterior lateral aspect of the ceratohyal and insert on soft tissues medially to control fine movements of the tongue. The levator hyoideus muscle, originating on the posterolateral chondrocranium and inserting on the cartilage of the posterior ceratohyal, acts with the interhyoideus muscles to move the entire hyoid apparatus forwards and pushes the tongue, supported by the basihyal cartilage, into the space between the mandibular bones to facilitate suctorial actions of the jaws and draw food into the mouth. The paired geniocoracoideus muscle, originating on the pectoral girdle and inserting ventrally on the hypohyal cartilages and anterior ceratohyal bones, and the rectus cervicis muscle, also paired, originating on the pectoral girdle and inserting on the dorsal surface of the hypohyal cartilages, moves the hyoid apparatus into a resting position.
1. Introduction
Modern lungfishes (Osteichthyes:Dipnoi) fall into two groups. The living Australian lungfish, Neoceratodus forsteri [1], is the only living representative of the Neoceratodontidae [2] that separated from other dipnoans in the Triassic [3], and is unusual in many ways. The Australian lungfish and close fossil relatives form a natural group, similar to the Permian genus Sagenodus [4, 5], but not to the other group of living lungfishes, which includes the South American lungfish, Lepidosiren paradoxa, and four species of African lungfish, classified in the genus Protopterus. These species comprise the Lepidosirenidae [6, 7]. Fossil genera can be aligned with one or the other group, based on certain characteristics [4] of the cranial and post cranial skeletons. Experts do not always agree on how the fossil lungfish are divided [4, 5, 8] but the living groups, assigned to the Neoceratodontidae or to the Lepidosirenidae, can easily be distinguished from each other, based on the dentition, structures of the skull and mandible, arrangements of the head musculature and characters relating to the life style and habitats of the species.
The dentition of lepidosirenids is highly refined and specialised, and includes a hard tissue, petrodentine, rare among dipnoans and absent in N. forsteri [9, 10]. The lepidosirenid dermatocranium is heavily ossified, with certain elements like the exoccipital bone exposed on the posterior skull, and a cranial (occipital) rib that can move and is placed in advance of the pectoral girdle [11, 12. 13]. This is completely unlike the structure present in N. forsteri, where the first rib of the trunk series [14], also known as the cranial or occipital rib, is immotile, articulating on the posterior chondrocranium via an amphiarthrosis, situated behind the pectoral girdle and embedded in the epaxial and hypaxial musculature of the trunk [15, 16]. The exocciptal bone of adult N. forsteri is enclosed in the posterior chondrocranium of adult fish and does not move. In keeping with differences in the skeleton, the arrangement of the muscles in the head differs in the two groups as well. Origins and insertions of the muscles in the head of N. forsteri, and their actions, are described in this paper.
2. Materials and Methods
2.1. Histology
Insertions, origins and potential actions of the muscles associated with the quadrate, mandible and hyoid apparatus in N. forsteri were analysed using two series of serial sections of juvenile lungfish, one of stage 53 and a second of stage 57. Stages follow Kemp [17, 18]. Specimens were embedded in Technovit, sections were cut at 3µm, and stained with Toluidine blue in buffer. Eggs and embryos were collected from the Brisbane River before the environment was irretrievably damaged by drought and flooding of the river [19], and raised to the required stages in the laboratory.
2.2. Morphology
The morphology of the jaws, hyoid apparatus, the exoccipital bone and the cranial ribs in the whole fish were analysed using an Alcian blue/Alizarin red stained specimen of stage 57 as well as adult fish [20].
3. Results
Elements of the mandible in young lungfish appear early in development, soon after hatching, with the blastemata of future skeletal structures, such as the ceratohyal, present by stage 43. Meckel’s cartilage forms on either side of the mandible at stage 44, fusing in the midline at stage 45, when the paired ceratohyal cartilages are well developed [18]. Hypohyal cartilages and the unpaired basihyal form slightly later, and complete the hyoid apparatus. Prearticular, vomerine and pterygopalatine dentitions appear by stage 45, as well as the transient dentary teeth, and the supporting bones by stage 46. Jaws are moveable by stage 46 when the hatchling begins to feed [21], initially by the active prehension of prey animals, aided by the presence of the dentary tooth plates, which carry sharp cusps at this stage of development, and the medial symphyseal tooth, which helps to prevent the escape of prey [22]. Feeding activities more usually found in the adult, which emphasise suction and not active prehension of prey, develop when the symphyseal tooth disappears at stage 51 [18], in line with the growth and increased function of the tongue. Over the lifetime of the fish, the dentition undergoes changes, with progressive development from small sharp cusps to catch and hold prey, to a tooth plate more suitable for crushing and grinding [23].
Meckel’s cartilage and the quadrate do not ossify, but are supported by bones of the jaws, the prearticular, which carries the lower tooth plate, and the angular in the lower jaw, and the pterygopalatine, with the upper tooth plate, and the parasphenoid in the upper jaw. The articulation between the quadrate and Meckel’s cartilage is bicondylar [18] and movements of the lower jaws permit rotational grinding of food by crushing between the upper and lower dentitions. The upper tooth plates and the pterygopalatine bone are fixed on the ventral surface of the chondrocranium, and do not move. The vomerine tooth plates, situated anteriorly in the mouth, just inside the upper lips, are unopposed and assist in the capture of prey as the fish draws material into the mouth.
Perichondral ossification of the ceratohyal begins at stage 46, and is well advanced by stage 50, when the young fish is able to take up an independent existence and leave the shelter of the water plants where it first hatched several weeks ago. The unpaired basihyal cartilage, supporting the pointed tongue, develops later, anterior to the hypohyal cartilages. It remains in articulation with these two elements. The basihyal and the hypohyals do not ossify, and the ceratohyal retains anterior and posterior cartilaginous extremities, as well as a core of cartilage, partially ossified by trabecular bone, throughout life. The hypohyals and ceratohyals lie in connective tissues within the curve formed by Meckel’s cartilage, below the oral epithelium shown in figures 1 and 2.. Both elements are moveable, as is the basihyal, which supports the tongue, and extends beyond the block of tissue surrounding the hypohyals and the anterior ceratohyal is shown in figure 1.
The tongue has considerable mobility within the oral cavity. When it moves forward during the active phase of suction, the tongue completely seals the space between the prearticular bones of the mandible below the anterior ridges of the tooth plates. When the hyoid apparatus is at rest, and not involved in suctorial movements, the whole element is drawn back by powerful muscles, and lies behind Meckel’s cartilage.
The coracoid, a cartilaginous, medial, unpaired element of the pectoral girdle, and involved in the origin of the depressor mandibulae and part of the geniocoracoideus muscles, forms early, along with the clavicle and cleithrum, thin curved bones that make up a major part of the pectoral girdle [24]. The occipital rib behind the pectoral girdle appears at stage 50. This element, the first of the ribs of the trunk and the largest in the series, is originally laid down in cartilage, and later ossifies perichondrally, while retaining cartilage at each extremity. The second rib of the trunk series is also enlarged compared with more posterior ribs. Trunk ribs, including the occipital rib, articulate with processes of cartilage on the posterior chondrocranium and the notochord. The joints are amphiarthroses and the ribs may support the musculature but they cannot move independently [15]. Muscles surround the ribs, but are not attached.
The lower tooth plates are ankylosed to the prearticular bone on the inner surface of Meckel’s cartilage in the mandible, supported by the angular and the splenial bones on the outer surface of Meckel’s cartilage. Between the two prearticular bones is a wide gap. The most anterior ridge of the upper tooth plates fits within the gap dorsally during mastication, but does not occlude it. The hyoid apparatus of N. forsteri consists of five elements, all based on cartilage and all in articulation. The paired ceratohyal is large, sheathed in bone but retaining a core of cartilage, which extends beyond the bone at both ends. The posterior extension of this element carries the muscles that work to move the hyoid apparatus forwards, and the anterior extension articulates with the small spherical hypohyals, also paired, consisting entirely of cartilage. The single medial basihyal, also entirely composed of cartilage, articulates between the two hypohyals. When the hyoid apparatus is moved forwards, the tongue completely seals the gap between the two prearticular bones.
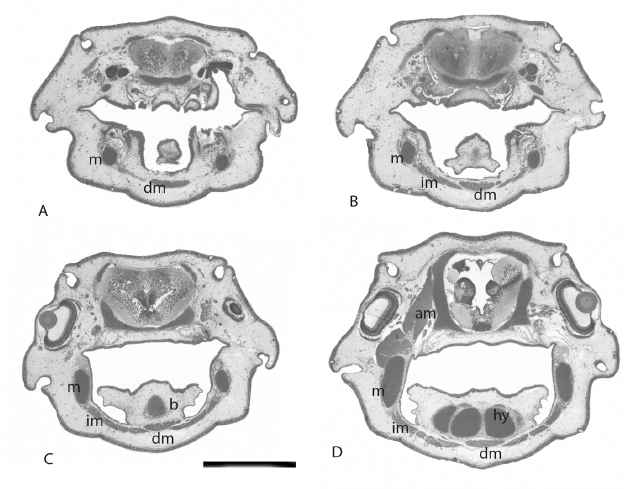
Development of the muscles that control jaw and hyoid movements proceeds alongside the appearance of skeletal elements. They begin to separate from the anterior myotomes by stage 41 [25, 26] and are functional when the hatchling starts to feed at stage 46. The muscle responsible for the opening of the mouth is the depressor mandibulae, a long, flat muscle, clearly visible, running ventrally along the floor of the oral cavity is shown in figure 1 and 2. It originates on the coracoid cartilage and on the clavicle, and inserts on the prearticular bones, covering the symphysis between the two bones. As it passes along the floor of the oral cavity, it lies close to but ventral to the geniocoracoideus muscle is shown in figure 1 and 2. Originating medially on the prearticular bones on either side, behind the tooth plates and reaching almost as far as the articulation with the quadrate, is a series of intermandibularis muscles is shown in figure 1 and 2, which have oblique fibres and insert on tissue in the mid line of the ventral oral cavity below the depressor mandibulae. Sub-terminal rotational grinding movements of the lower jaw tooth plates during mastication are carried out by the intermandibularis muscles. They are attached to the prearticular bones behind and below the tooth plates on each side, and reach back along the mandible close to the articulation with the quadrate is shown in figure 1 and 2. These muscles are responsible for the complex masticatory movements of the lower jaw dentition, mediated by the bicondylar articulation of the jaws. The upper jaw and the upper tooth plates are incapable of movement.
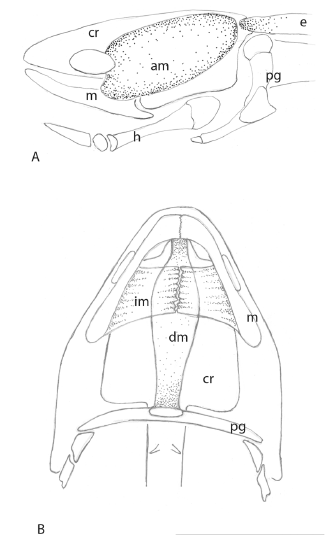
The mouth is closed by the action of the paired, massive adductor mandibulae muscles, which originate on the dorsolateral chondrocranium, and reach as far anteriorly as the posterior surface of the orbit is shown in figure 1 and 2. This muscle extends from the chondrocranium on each side and extends almost to the edge of the posterior chondrocranium is shown in figure 2A, close to the insertion of the epaxial muscles of the trunk. It is not attached to any osseous component of the head, only to the chondrocranium. This muscle is inserted on the posterior aspect of Meckel’s cartilage is shown in figure 1 and 2. Behind the adductor mandibulae are the anterior epaxial muscles of the trunk, also originating on the chondrocranium is shown in figure 2A.
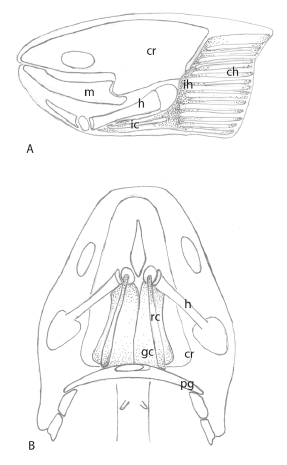
Muscles associated with the hyoid apparatus are larger than the muscles attached to the prearticular bones. The interhyoideus muscles, also in blocks and with oblique fibres, originate on the lateral surface along the shaft of the ceratohyal, and are inserted medially in tissues that surround the ceratohyal is shown in figure 3 and 4. These muscles are associated with the levator hyoideus muscle, which originates on the chondrocranium below the otic capsules, passes behind the opercular bones and inserts on connective tissues surrounding the lateral surface of the posterior ceratohyal cartilage is shown in figure 3 and 4. A series of strips of muscle extend behind the levator hyoideus, and pass posteriorly within the operculum, to make up the constrictor hyoideus muscles is shown in figure 3 and 4. Interhyoideus muscles may contract to move the ceratohyal from side to side, and these muscles, aided by the levator hyoideus and the constrictor hyoideus muscles that extend from the interhyoideus is shown in figure 4, push the whole hyoid apparatus forwards, a movement that causes the basihyal to be inserted into the gap between the prearticular bones and Meckel’s cartilage, sealing the space completely. This action facilitates suction of material into the oral cavity. In effect , the hyoid apparatus is enclosed by muscles, almost a single element but with two parts is shown in figure 4. Slow movements of the opercular folds to maintain a flow of water over the gills arise from separate contractions of the constrictor hyoideus, and movements of these muscles aid the action of the levator hyoideus. Levator hyoideus and constrictor hyoideus muscles pass behind the opercular bones and are not attached to these bones.
The geniocoracoideus muscle, which retracts the hyoid apparatus, is a massive paired muscle that is inserted on the base of the hypohyals and the anterior ceratohyal. Ventrally this muscle originates on the pectoral girdle, behind the insertion of the depressor mandibulae is shown in figure 4. Above the hypohyals and the shaft of the ceratohyal is the rectus cervicis muscle is shown in figure 4, attaching dorsally on each side of the hyoid apparatus. The geniocoracoideus and the rectus cervicis draw the hyoid apparatus back, and hold it in a resting position.
The process of suction, of food, air, water or mud, into the oral cavity is as follows. The constrictor hyoideus, the interhyoideus, and levator hyoideus muscles contract, and push the hyoid forwards, pressing the tongue, supported by the basihyal, into the gap in the mandible between the prearticular bones. The depressor mandibulae contracts, lowers the mandible and opens the mouth. Material is drawn into the oral cavity. The constrictor hyoideus, interhyoideus and the levator hyoideus muscles relax and the geniocoracoideus and rectus cervicis muscles contract, drawing the hyoid apparatus back into the resting position. The adductor mandibularis contracts, closing the mouth. Intermandibularis muscles move independently to break up the items of food.
Muscles surrounding the cranial rib are not attached to the rib, which cannot move. The only influence the cranial rib can have on the oral cavity and the hyoid apparatus is to anchor the elements of the oral cavity and hyoid apparatus, in conjunction with the pectoral girdle and the hypaxial musculature. This rib is not involved in suctorial actions of the jaws and hyoid apparatus.
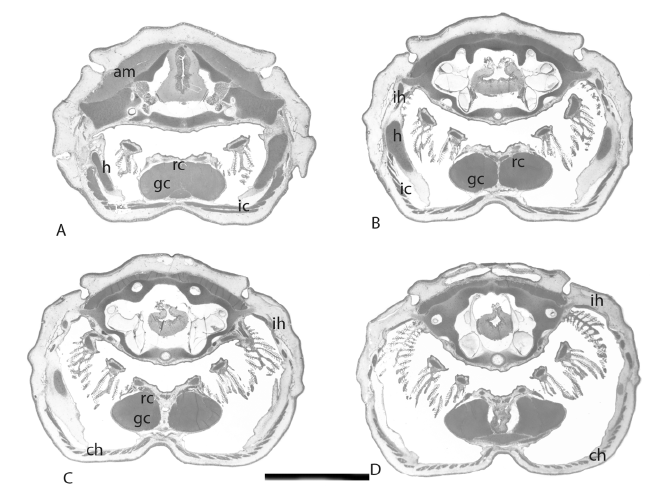
Figure 3: Sections of the stage 57 juvenile showing muscles that operate the hyoid apparatus. am, adductor mandibularis muscle, ch constrictor hyoideus, gc geniocoracoideus, h hyoid (ceratohyal), ic interceratohyal muscle, ih interhyoideus muscle, rc rectus cervicis. Scaler bar = 1 cm.
4. Discussion
Fossil dipnoans should not be seen as fundamentally different from living lungfish, and comparisons of functional anatomy among the different groups will enhance understanding of all dipnoans and their environments. There are many similarities in both ancient and living lungfish, as well as many differences, and comparisons can be both helpful and revealing. It is, after all, an ancient lineage, beginning in the Devonian and still with living representatives [7, 24, 27, 28].
The two groups of living lungfishes, neoceratodontids and lepidosirenids, are not the same, and similar differences can be traced in their closest fossil relatives, even as far back as the Palaeozoic [4, 8, 29, 30]. Mostly, the discrepancies relate to the structure of the skull and the dentition, but they can also be found in the muscular systems of the two groups [31, 32]. The skulls and jaw bones of N. forsteri, L. paradoxa and species of Protopterus contain the same elements, such as a cartilaginous chondrocranium, calvarial bones, tooth plates ankylosed to the jaw bones, a hyoid arch skeleton, pectoral girdle and cranial ribs [11, 12, 13, 15, 18, 33]. However, the arrangement, distribution and function of these elements differs in the two groups. Names of the elements of the skull roof differ depending on the interests of the authors describing the structures, with some using names that were originally designed for other vertebrates [8, 13, 26, 30, 31]. Other authors adopt a system of letters and names more appropriate for dipnoans [18, 33, 34].
The degree of ossification of the skull bones in the two major groups of living lungfishes differs, as does the shape and to a certain extent the function of the elements. In N. forsteri, the skull is essentially composed of a cartilaginous chondrocranium, with a calvarium consisting of thin bones in articulation with each other, separate from the chondrocranium, and the adductor mandibularis which closes the jaws, and the most anterior of the epaxial muscles, both originating on the posterior chondrocranium. The upper tooth plates are attached to the pterygopalatine bone, supported by the parasphenoid, and the vomer, situated just inside the upper lip, carries the small vomerine tooth plates. An ascending process from the pterygopalatine bone articulates with a lateral bone of the calvarium, designated the JLM bone [18], or the supraorbital bone [31]. This represents the only articular link between the calvarial bones and the underlying chondrocranium in this species. Meckel’s cartilage, the major element of the mandible, remains cartilaginous throughout the life of the fish, as does the quadrate, an extension of the persistent chondrocranium.
According to Bemis [31], Neoceratodus forsteri does not have a depressor mandibulae muscle, to open the mouth, but this is not the case. Analysis of serial sections shows that a depressor mandibulae muscle is present, in the oral cavity between the two rami of the prearticular bone that carries the tooth plates, lying below the geniocoracoideus muscle, inserting in a more anterior position on the prearticular bones and originating on the bones of the pectoral girdle. This is not in the same position as the depressor mandibulae in Lepidosiren and Protopterus [31].
The living Australian lungfish is exceptional in that some of the muscles of the jaws and tongue are inserted only into soft tissues, not on bone or cartilage. Muscles in the jaw in N. forsteri appear to be rudimentary [25. 26] but the movements that are carried out are surprisingly versatile. Two muscles operate to open and close the jaws, the depressor mandibulae and the adductor mandibularis, and the lower tooth plates carry out subterminal grinding using small mandibular muscles attached to the prearticular bone. Large and powerful muscles in the throat move the hyoid apparatus back into a resting position, and a series of muscles attached to the ceratohyal push the tongue forwards to seal the gap between the lower jaw bones when the fish carries out suctorial movements of the oral cavity. In addition, movements of the operculum depend on the actions of the constrictor hyoideus muscles behind the ceratohyal, and associated with the interhyoideus muscle, to facilitate respiration by drawing water over the gills.
Lepidosirenids utilise different structures and mechanisms. The articulation between the upper and lower jaws is restrictive, not bicondylar as it is in N. forsteri, and is only capable of moving up and down. They have no basihyal, and no gap between the tooth plates of either the upper or lower jaws. Muscles of lepidosirenids include the same elements found in N. forsteri but the arrangements differ [31, 32], as do the size and relative importance of the muscles.
5. Conclusions
- Two muscles operate to open and close the jaws in forsteri, the depressor mandibulae and the adductor mandibularis, and the lower tooth plates carry out subterminal grinding using small intermandibular muscles attached to the prearticular bone.
- A series of muscles attached to the ceratohyal push the tongue forwards to seal the gap between the lower jaw bones when the fish carries out suctorial movements of the mouth. These include the muscles attached to the ceratohyal bone, as well as elements of the interhyoideus and the constrictor hyoideus muscles. Large and powerful muscles in the throat, the geniocoracoideus and rectus cervicis muscles, move the hyoid apparatus back into a resting position.
- In addition, movements of the operculum depend on the actions of the constrictor hyoideus muscles behind the ceratohyal, and associated with the interhyoideus muscle, to facilitate respiration by drawing water over the gills.
Data Availability
Data on which this article is based are included in the text.
Author Contribution
The research was carried out by the author. The technical assistance of Tina Chua, who made the serial sections, is gratefully acknowledged.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgment
This research was carried out with permission from the University of Queensland Animal Ethics Committee, approval number CMM/013/03/ARC and the Queensland Fisheries Management Authority, permit number PRM03012K.
- G. Krefft, “Description of a gigantic amphibian allied to the genus Lepidosiren, from the Wide-Bay District, Queensland.” Proceedings of the Zoological Society of London. 1870, 221 – 224. 1870.
- R. S. Miles, “Dipnoan (lungfish) skulls and the relationships of the group: a study based on new species from the Devonian of Australia.” Zoological Journal of the Linnean Society. 61, 1–328. 1977.
- L. Cavin, V. Suteethorn, E. Buffetaut, H. Tong, H., A new Thai Mesozoic lungfish (Sarcopterygii, Dipnoi) with an insight into post-Palaeozoic dipnoan evolution. Zoological Journal of the Linnean Society, 149, 141–177. 2007.
- A. Kemp, L. Cavin, G. Guinot, “Evolutionary history of lungfishes with a new phylogeny of post-Devonian genera.” Palaeogeography, Palaeoclimatology, Palaeoecology, Paleontology. 4, 457–470. 2017.
- H.-P. Schultze, J. Chorn, “The Permo-Carboniferous genus Sagenodus and the beginning of modern lungfish.” Contributions to Zoology. 67, 9–70. 1996.
- R. Owen, “Description of the Lepidosiren annectens.” Transactions of the Linnean Society.18, 327-361. 1839.
- H.-P. Schultze, “Fossilium Catalogus 1: Animalia, pars 131, Dipnoi.” Kugler Publications, Amsterdam, New York. 1992.
- T. J. Challands, et al., 2019. “A lungfish survivor of the end-Devonian extinction and an Early Carboniferous dipnoan radiation.” Journal of Systematic Palaeontology, https://doi/10.1080/14772019.2019.1572234. 2019.
- L. Lison, “Recherches sur la structure et l’histogenese des dents du poisons dipneustes.” Archives de Biologie. 52, 279-320. 1941.
- A. Kemp, “Petrodentine in derived dipnoan dentitions.” Journal of Vertebrate Paleontology. 21, 422-437. 2001.
- W. E. Agar, “The development of the skull and visceral arches in Lepidosiren and Protopterus.” Transactions of the Royal Society of Edinburgh. 45, 49-64. 1906.
- T. W. Bridges, “On the morphology of the skull in the Paraguayan Lepidosiren and in other Dipnoids.” Transactions of the Zoological Society of London, 14, 325-376. 1898.
- K. E. Criswell, “The comparative osteology and phylogenetic relationships of African and South American lungfishes (Sarcopterygii: Dipnoi).” Zoological Journal of the Linnean Society. 174, 801-858. 2015.
- H. S. Baird, “The occipital bones of the Dipnoi.” Proceedings of the Royal Society of Victoria. 35 (N.S.), Part II,115-116. 1923.
- A. Kemp, “Adaptations to life in freshwater for Mioceratodus gregoryi, a lungfish from Redbank Plains, an Eocene locality in southeast Queensland, Australia.” Alcheringa: An Australasian Journal of Palaeontology, 42, 305-310. 2018.
- A. Kemp, “Osteogenesis in the Australian lungfish. Neoceratodus forsteri (Osteichthyes:Dipnoi).” Australian Journal of Zoology. https://doi.org/10.1071/ZO22004. 2022.
- A. Kemp, “The embryological development of the Queensland lungfish, Neoceratodus forsteri (Krefft).” Memoirs of the Queensland Museum. 20, 553-597. 1982.
- A. Kemp, “Ontogeny of the skull of the Australian lungfish, Neoceratodus forsteri (Osteichthyes: Dipnoi).” Journal of Zoology. 248, 97-137. 1999.
- A. Kemp, “Changes in the freshwater environments of the Australian lungfish, Neoceratodus forsteri, in south east Queensland, and implications for the survival of the species.” Proceedings of the Royal Society of Queensland, 124, 121 – 135. 2020.
- W. L. Kelly, M. Bryden, “A modified differential stain for cartilage and bone in whole mount preparations of mammalian foetuses and small vertebrates.” Stain Technology, 58, 131-134. 1983.
- A. Kemp, “The role of epidermal cilia in development of the Australian lungfish, Neoceratodus forsteri (Osteichthyes: Dipnoi).” Journal of Morphology, 228, 203-221. 1996.
- A. Kemp, “Marginal tooth bearing bones in the lower jaw of the Recent Australian lungfish, Neoceratodus forsteri (Osteichthyes:Dipnoi).” Journal of Morphology, 225, 345- 355. 1995.
- A. Kemp, “Growth and hard tissue remodelling in the dentition of the Australian lungfish, Neoceratodus forsteri (Osteichthyes: Dipnoi).” Journal of Zoology. 257, 219-235. 2002.
- E. Jarvik, “Basic structure and evolution of vertebrates.” Volume I. Academic Press, New York. 1980.
- F. H. Edgeworth, “On the development of the hypobranchial, branchial and laryngeal muscles of Ceratodus, with a note on the development of the quadrate and epihyal.” Quarterly Journal of Microscopical Science, new series. 67, 325-368. 1923.
- F. H. Edgeworth, “The Cranial Muscles of Vertebrates.” Cambridge: Cambridge University Press. 1935.
- R. H. Denison, “Early Devonian lungfishes from Wyoming, Utah and Idaho.” Fieldiana: Geology, 17, 353 – 413. 1968.
- W. Gross, “Ueber Crossopterygier und Dipnoer aus dem Baltischen Oberdevon im Zusammenhang einer vergliechenden Untersuchung des Porencanalsystems palaozoicher Agnathen und Fische.” Kungliga Svenska Vetenskaps Akademiens Handlingar. 5, 1 – 140. 1956.
- T. J. Challands, et al. “Mandibular musculature constrains brain –endocast disparity between sarcopterygians.” Royal Society Open Science, 7, 200933. http://dx.doi.org/10.1098/rsos.200933. 2020.
- J. D. Pardo, et al. “An exceptionally preserved transitional lungfish from the Lower Permian of Nebraska, USA, and the origin of modern lungfishes.” PLoS ONE 9, e108542. 2014.
- W. E. Bemis, “Feeding systems of living dipnoi: anatomy and function.” Journal of Morphology, supplement 1 , 249-275. 1987.
- W. E. Bemis, G. V. Lauder, “Morphology and function of the feeding apparatus of Lepidosiren paradoxa (Dipnoi).” Journal of Morphology, 187, 81- 108. 1984.
- A. Kemp, “Skull Structure in Post-Paleozoic Lungfish.” Journal of Vertebrate Paleontology, 18, 43-63. 1998.
- A. S. Romer, “The dipnoan cranial roof.” American Journal of Science. 32, 241-256. 1936.
No related articles were found.
