AI-Powered Decision Support in SAP: Elevating Purchase Order Approvals for Optimized Life Sciences Supply Chain Performance
Journal of Engineering Research and Sciences, Volume 4, Issue 8, Page # 41-49, 2025; DOI: 10.55708/js0408005
Keywords: SAP, S/4 HANA, Artificial Intelligence (AI), Purchase Order Approval, Procurement Controls, Supply Chain Management (SCM), Life Sciences, Trend Analysis, GxP Compliance, Regulatory Compliance, Supply Chain Resilience, Supply Chain Optimization, Digital Transformation
(This article belongs to the Section Artificial Intelligence – Computer Science (AIC))
Export Citations
Cite
Apelagunta, V. and Tatavandla, V. R. (2025). AI-Powered Decision Support in SAP: Elevating Purchase Order Approvals for Optimized Life Sciences Supply Chain Performance. Journal of Engineering Research and Sciences, 4(8), 41–49. https://doi.org/10.55708/js0408005
Vinil Apelagunta and Vishnuvardhan Reddy Tatavandla. "AI-Powered Decision Support in SAP: Elevating Purchase Order Approvals for Optimized Life Sciences Supply Chain Performance." Journal of Engineering Research and Sciences 4, no. 8 (August 2025): 41–49. https://doi.org/10.55708/js0408005
V. Apelagunta and V.R. Tatavandla, "AI-Powered Decision Support in SAP: Elevating Purchase Order Approvals for Optimized Life Sciences Supply Chain Performance," Journal of Engineering Research and Sciences, vol. 4, no. 8, pp. 41–49, Aug. 2025, doi: 10.55708/js0408005.
Resilient and compliant supply chains, while essential to the Life Sciences, depend heavily upon SAP systems to manage the complexities involved. The standard Purchase Order (PO) approval process in SAP is an important upstream control point in the supply chain, but seldom has the required intelligence needed to manage endorsed compliance (e.g., GxP) or to be proactive in supply chain risk mitigation. This paper offers an introduction to a proof of concept that demonstrates how an AI enabled, decision support solution that embeds into SAP processes and workflows can provide opportunities to transform this critical process and improve overall performance within the supply chain. Beginning with the evolution of SAP’s approval workflows, the paper updates the concepts around AI/ML applications for improving various supply chain functions, and situates intelligent automation as part of the strategic digital transformation landscape for Life Sciences. The paper establishes constructs to improve PO approvals through the embedding of AI contextually based insight to build in performance trend analysis of suppliers (e.g., delivery, quality) and contextually relevant compliance checks as part of the decision process. These and other safeguards can move the PO approval process from being predominately procedural to a more strategic control point, increasing supply chain visibility, resilience, compliance assurance, and operational performance relevant to the Life Sciences.
1. Introduction
In today’s unpredictable global markets, especially under regulatory requirements (GxP) in the Life Sciences sector, where patient safety, regulation, and product efficacy are responsibilities we cannot overlook [1], to enable resilient, compliant, and efficient supply chain operations is not only necessary. The critical upstream supply chain processes of procurement and the subsequent approval of purchase orders (POs) are crucial control points that have a significant impact on downstream performance. The performance of such downstream processes could extend to manufacturing continuity, inventory levels, quality of finished product, and most importantly, on-time delivery of finished product [2], all of which involves approval of purchase orders (POs). We rely increasingly on Enterprise Resource Planning systems (predominantly SAP S/4HANA) and often embedded with specialized systems such as those offers for Integrated Business Planning (IBP), Quality Management and Business Network, most of which are interconnected by the same database, to manage the flow of such processes throughout the supply chain. Nevertheless, the traditional PO approval mechanisms in SAP, despite efforts to enhance flexibility, traditionally remain procedure-focused [3]. They usually lack the situational intelligence to determine where suppliers might present a supply chain risk— such as reliability de-
clines, quality declines— or the ability to rigorously enforce compliance with any stringent Life Sciences quality agreement or GxP requirements [4, 5]. This analytical gap at an upstream control point can introduce risks throughout the supply chain and open the door to costly disruptions, serious quality failures that impact patient safety, or compliance failures.
To tackle many supply chain issues harnessing the transformative potential of Business Process Automation (BPA), Digital Transformation and advanced technologies (Artificial Intelligence (AI) and Machine Learning (ML)), across the end-to-end value chain must be a key part of the solution [2, 6]. This paper will examine these trends, in the context of SAP purchase order (PO) approvals. More specifically, this paper will:
- Track the historical evolution of SAP PO approval workflows, identifying their historical weakness in enabling dynamic supply chain risk assessment.
- Review prevalent applications of AI/ML now impacting supply chain activities that are managed through SAP and set the context for advanced analytics.
- Consider the use cases of AI/ML techniques in the context of supply chain digitalization and a BPA strategy.
- Provide an aspirational two future states, embedding advanced analytics (specifically supplier performance trend analysis and context relevant quality/compliance analytics) in the PO approval decision process, to strengthen upstream controls, for improved supply chain effectiveness.
The aim is to shine a light on the continuum from basic workflow automation to real intelligent decision support, then find examples of how we can take an essential administrative action and conceptualize it as a control point that is more informed, efficient, and strategic in the world of contemporary procurement, particularly the Life Sciences supply chain.
2. Literature Review
2.1. Evolution of SAP Purchase Order Approval Workflow
The approval of purchase orders (POs) in SAP’s Materials Management (MM) module is a significant control within the overall supply chain’s Procure-to-Pay cycle. Over the years, SAP systems have leveraged configurable, however, inflexible, rule-based methods called ’Release Strategies’ (ME28/ME29N) to route POs for approval based on predetermined criteria like value, material group or plant. As summarized in Figure 1, while helpful for basic policy enforcement, these strategies are limited in authority and focus on authorizations limited to factual invariant conditions. More recently, SAP has the ’Flexible Workflow’ architecture in S/4HANA, which has greater flexibility in specifying multi-step approval processes, dynamic approval authori- ties (based on roles or logic specified through BAdIs) and time frames [3, 7]. Yet even with more flexible frameworks, the standard decision-making functions of these workflows still rely heavily on procedural notification to approvers whose documents are routed according to configured rules, rather than providing contextual analysis, or data-driven insights to support the approvers’ decision-making process [3].
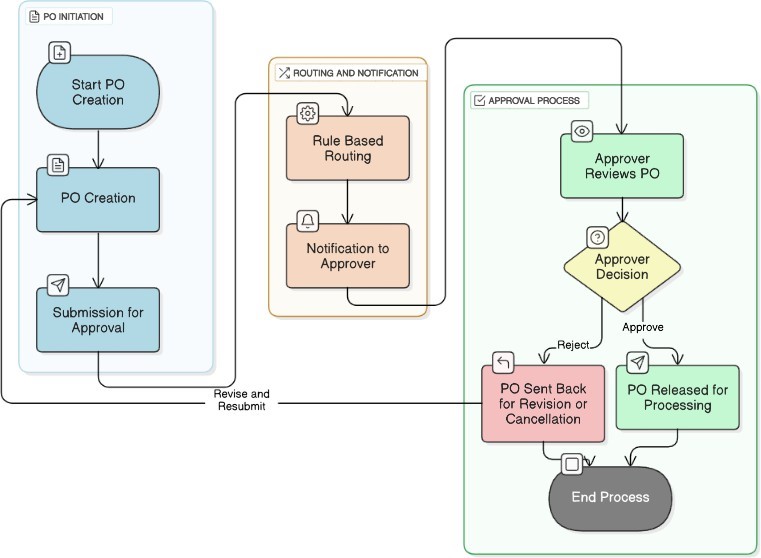
2.2. Leveraging AI and Machine Learning in SAP-Driven Life Sciences Supply Chains
Organizations are not only taking advantage of typical workflow capabilities, but also increasingly utilizing Artificial Intelligence (AI) and Machine Learning (ML) to build efficiency and predictive capabilities across critical supply chain processes handled in SAP systems, which is important in a stressful Life Sciences environment. One critical area is improving supplier management, where AI/ML can go well, beyond simple financial checks into evaluating historical delivery performance (e.g. OTIF rates), quality records (e.g. batch acceptance rates, audit outcomes), GxP standards, regulatory compliance certifications, and accepted historical rates to establish dynamic risk scores related to possible impacts on supply chain performance or compliance failures [2, 8].
In addition, AI/ML applications are being adopted throughout the wider Life Sciences supply chain: increasing the accuracy of demand forecasting in platforms like SAP IBP in order to assure product availability [2]; optimizing inventory levels, especially for materials requiring special storage conditions (e.g., temperature) or products with short shelf-life [2]; enabling predictive quality analytics by combining data from manufacturing, quality management (QM) and laboratory information management system (LIMS) in order to proactively predict future batch deviations [9]; improving logistics agility, including route optimization, or the prediction of transport delays and risks [4, 10]; and AI/ML can address substantial compliance requirements including analyzing traceability data (e.g., serialization) or the automation of some aspects of regulatory documentation management . Many academic surveys report on AI applications used in supply chain management, but usually focus on using resilience for creating risk management strategies that are important in Life Sciences sector [4].
AI/ML provides supply chain efficiency across the Procure-to-Pay (P2P) cycle by automating functions like invoice matching [11] and utilizing complex multi-way matching rules (PO, Goods Receipt, Quality Inspection) [11] to identify anomalies related to errors or fraud [11]. Typically used in conjunction with these intelligent applications, Robotic Process Automation (RPA) technology provides solutions for repetitive, high-volume, rule-based functions [10]. RPA sometimes blends into AI technologies, enabling Intelligent Process Automation (IPA) [10]. Although AI/ML applications enhance visibility, efficiency, and predictive capabilities in the supply chain, many are limited to specific functional areas and largely rely on structured data [12]. Integration of these outputs without displacing them, expanding AI logic capabilities to support complex, cross- functional supply chain decisions, such as the PO approval situation described later, is still work in progress and opportunity.
2.3. The Strategic Role of Digital Transformation in Life Sciences Supply Chains
The journey on established workflows within SAP (which are discussed in “Evolution of SAP Purchase Order Approval Workflows”) and the usage of specific AI/ML applications (which are discussed in “Leveraging AI and Machine Learning in SAP-Driven Life Sciences Supply Chains”) are important building blocks on a larger strategic objective: Business Process Automation (BPA) and Digital Transformation fundamentally changing Life Sciences supply chain operations. This transformational ambition is beyond just incremental improvements, but redesigning supply chain processes from end-to-end – planning, sourcing, manufacturing, quality, and logistics – to support better operational efficiency, maintain strong GxP compliance, foster greater end-to-end visibility and traceability, enhance supply chain resilience from disruptions, better manage costs, and to optimize the overall function of the supply chain to generate strategic value, all while preserving product quality and patient safety [1, 4, 5].
In this context of supply chain transformation, technologies marketed by vendors like SAP, such as their ’Flexible Workflow’, are designed to offer flexible infrastructures to enable coordination of complex processes with many internal or external participants. Robotic Process Automation (RPA) tackles high-volume, rule-based tasks in various activities [10]; specific AI/ML solutions (described above) provide predictive when the use of pattern recognition is needed to better manage supply chain risk with forecasting accuracy, quality control, or logistics [2, 4, 9]. The overall aim is increased integrated, intelligent, data-driven supply chain workflows to remove manual bottlenHosseinOrdibazar2025, ecks, secure the same data accuracy necessary for regulation oversight, and exploit real-time-analytics to quickly inform decisions across the supply chain life-cycle [13].
However, in order for the full promise of digital transformation in the complex and highly regulated Life Sciences value chain to be realized, a number of considerable barriers must be addressed, such as orchestrating together the many disparate systems (e.g., ERP, MES, QMS, LIMS, logistics interface), ensuring consistent, governed data quality across the value chain, executing organizational change management across siloed functions, and scaling from incremental improvements demonstrated during pilot projects to comprehensive, end-to-end intelligent supply chain automation that embodies advanced levels of reasoning and adaptive management [14, 15, 16].
It is essential to recognize that there are many commercial offerings, such as SAP’s own Ariba and Spend Control Tower, that are now including AI as part of providing high- level spend analytics and supplier risk scorecards. These are effective solutions for purposes like strategic spend categorization, and identifying high-level supplier risks. But the framework which is put forward in this paper has an advantage: it puts highly contextual, real-time trend analysis directly into the transactional PO approval workflow. Unlike high-level dashboards that analytics providers have developed, we provide not just situational or landscape awareness, but micro intelligence (e.g., “Is the quality of this specific material from this supplier trending down in the last month?”) , and most importantly, we provide it to the decision-maker exactly at the moment that they use it at a GxP-relevant control point. Operationalizing intelligence for front-line decision making and not just strategic decision making is a key feature identifying our contribution.
Operationalizing intelligence for key decisions at the frontline, and not exclusively at the strategic level, is proprietary to our offering. This distinction mirrors the character of Procurement 4.0, moving from the procurement of duel to procuring in a more intelligent way and ultimately for the transformative process. [17].
3. A Proposed Framework: AI-Powered Contextual Intelligence for Strategic PO Approvals
The purpose of the strategic supply chain transformation previously outlined is to more than simply provide a procedural checkpoint with respect to the SAP purchase order approval process (PO). The intention is to create a knowledgeable, data-driven point of decision that enables effective management of Life Sciences supply chain resilience, compliance, and performance [18]. This means inserting capabilities that use advanced analytics — AI/ML based — into the workflow so that approvers receive actionable intelligence related to the PO, and the supply chain context surrounding it.
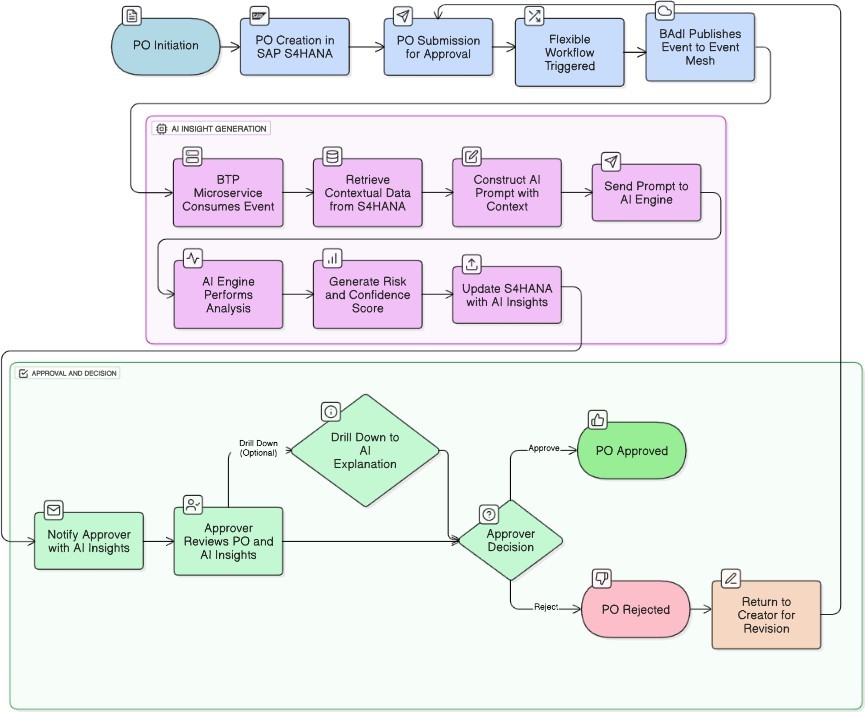
Advanced decision support should provide performance data in context and trends [13, 19]. For example, if the system examines the historical delivery data of the items on the current PO, it could highlight if, while recently restrictions on single items were only recent, the delays appear to be a sign of a generally worsening trend that has ingress to disruption of timelines in critical production or supplies of clinical trials. Just as the system should examine trends in product quality, what tends are we seeing in rejection rates, returns and notifications/CAPAs associated with Quality Management (QM) for these specific materials? Are the trends arising from a concerned review of data about even possible lapses in GxP compliance or risks to the integrity of a batch, based on the recent performance of this supplier? Most critically, the System will recognize and track initial quality issues to specific products/batches that appear on this PO, as well as determining if this supplier and material combinations are consistent with current validation statuses and if quality agreements are indicated where this is particularly essential in Life Sciences [5, 9]. This type of dynamic trend analysis will provide intelligence not typically achieved from scorecards of static supplier performance in places.
3.1. Conceptual Technical Architecture and Implementation Approach (Event-Driven)
In order to appropriately deliver the AI-powered PO approvals insights in a decoupled and resilient architecture, an event-based solution that uses SAP S/4HANA and the SAP Business Technology Platform (BTP) is a fundamentally sound approach aligned to clean core principles [20]. The example ’To-Be’ process flow is presented in Figure 2. The process would initiate from within the SAP S/4HANA Purchase Order approval workflow (e.g., Flexible Workflow). At some determinate step (e.g., at the approval step being ready to send assignment, or just prior to triggering a notification), we would implement a Business Add-In (BAdI) or enhancement spot within the workflow. This ABAP logic would be responsible for:
- Event Publication: An example for discussion is publishing a business event (e.g., “POApprovalInsightsReuired”) using any topic on SAP Event Mesh (running on BTP) or other enterprise messaging queue. The event payload would include some critical identifiable information for the Purchase Order (e.g. PO number, company code, etc.). Thus, the S/4HANA core process remains standard, with the extension point being unambiguous and upgrade safe.
A microservice, built and hosted on SAP BTP (or any other cloud platform of your choosing), would subscribe to the “POApprovalInsightsRequired” event. On encountering the event, this BTP service would first conduct the following steps:
- Contextual Data Retrieval: The BTP service would call secure APIs (e.g., OData services backed by pre-built Core Data Services (CDS) views) back to S/4HANA to retrieve the full breadth of data for context. The BTP service would retrieve historical supplier performance, quality management data (QM notifications, CAPA summaries), compliance status, batch info, and relevant document info for the PO items and supplier.
- Prompt Development & AI Engagement: The service would develop an optimized prompt for the designated AI engine (ideally a company-native or private instance of an LLM for data security). This prompt would combine the retrieved contextual data and instruct the AI to conduct risk assessments, trend analysis and generate the score, sub-metrics and A significant item in design consideration is the selection of the AI model. While traditional machine learning models (e.g. regression or time-series) could be very powerful and computationally efficient for performing analysis of structured performance data, the proposed framework is based on a Large Language Model (LLM) for a specific purpose: synthesis & explanation. The goal is to have a hybrid approach whereby a LLM acts as the reasoning engine using & interpreting many data types, including structured outputs of the traditional machine learning models, unstructured text in Quality Notification long-text fields, & codified GxP compliance rules [5, 21]. The uniqueness of LLM models comes from their ability to synthesize these data types into a cohesive narrative (that is human-readable) and actionable recommendation, and solve the “black box” question by providing clarity into why it provided the risk score.
- Asynchronous Contextual Insight Update: After receiving the AI analysis, the BTP service would then asynchronously update the relevant context in S/4HANA. This could happen by hitting a custom API exposed by S/4HANA (to update custom fields on the PO, custom tables or append to workflow attachments) or by saving those insights in a data store on BTP, which could be queried by S/4HANA or Fiori apps when the approval task was presented.
The PO approval notification (email or Fiori ’My Inbox’ item) would then be customized to retrieve and visualize AI-based insights, which could include a minor conditional branch in the notification generation or Fiori UI to check for existing insights before rendering, or perhaps a secondary notification/update if the insights are received after the original task is created.
For the risk analysis report, a similar event-driven, or direct API call, could be established. A user action (i.e., clicking a link in Fiori app) may create a new event for the BTP service, or directly call it to request the full report from the AI engine, which will ultimately be passed back to the user (i.e., email, Fiori UI, etc.).
To make the information consumable and actionable, the vision is to present insights directly in the approver’s standard interface (i.e., SAP Fiori app, notification email, etc.). This could be a summary “PO Risk/Confidence Score” [4], along with some key contributing sub-metrics (i.e., ’Recent On-Time Delivery Trend’, ’Quality/Compliance Flags (PO Items)’). For transparency, and to allow for exploratory dives, approvers would be able to quickly drill-down to a detailed report that explains how the score was derived, visualizes performance trends, and cites specific supporting data points or events (e.g., recent delivery delays affecting key production lines, specific QM notifications against PO items) [4, 13].
With such capabilities, the approval task could be quantitatively transformed from primarily an administrative check, to an informed risk-based decision. It would allow approvers to make decisions in more-informed, fast, and confident manners, while allowing the explicate identifica- tion of upcoming supply chain interruptions or compliance failures, and complete an important contribution to overall Life Sciences supply chain integrity, agility, and perfor- mance, and in doing so, the appropriate safeguarding of patient safety, and continuity of care [1, 5, 19].
3.2. Key Considerations for this Event-Driven Architecture
- Asynchronous Nature: Despite promoting decoupling and resilience, this architecture may contribute to latent time to insights. A formal Service Level Agreement (SLA) for insights needs to be defined (e.g., 95% of insights are received in 60 seconds of PO submission), in order to mitigate delays/models that may stall the approval process. The UI, e.g., the Fiori “My Inbox” application, needs to anticipate this and provide a status such as “AI insights are being generated…” to establish user expectations effectively.
- Complexity: The complexities of managing distributed transactions, ensuring you have eventual consistency, and monitoring event flows can be more complicated than using a synchronous model.
- Resilience & Scalability: This architecture is typically more resilient to failures in individual components and can scale components in the solution independently.
- Clean Core: With the use of BAdIs publishing events from S/4HANA, and using BTP for extensions, you are staying true to SAP’s clean core.
4. Simulated Case Study: AI-Powered Decision Support at ’Innovida Life Sciences’
To demonstrate the applied impact of the proposed framework and what it means in practice, this section presents a fictitious case study of a fictitious company, “Innovida Life Sciences”.
Scenario: Innovida Life Sciences is a mid-sized biopharmaceutical company that has a leading product, “Gerocept,” a temperature-sensitive biologic drug used in critical patient therapies. Innovida’s manufacturing process of Gerocept is based on the availability and quality of a critical raw material, in this case, “Stabilizer-7”, and associated tight quality and delivery processes as a GxP compliant product. Unfortunately, there have been sporadic delays in manufacture and delivery of Gerocept-linked to the performance of the company’s primary supplier of Stabilizer-7, “Global Bio- Reagents Inc” [4]. Such challenges are common in complex pharmaceutical supply chains, where AI and big data analytics are increasingly being leveraged for greater efficiency and risk mitigation [22].
4.1. The ’As-Is’ Process: A High-Risk PO Approval
A new Purchase Order (PO #4500012345) is generated in SAP S/4HANA for a transactional shipment of 1000L of Stabilizer-7. The PO now enters the standard flexible workflow for approval, as shown in general terms in Figure
- The approver who is a procurement manager will review the PO using the standard criteria: right material, right quantity, right price, and right cost center; and will see that the vendor, Global Bio-Reagents Inc., is on the approved vendor list. At this stage it looks pretty normal.
But there are important risk signals buried in separate SAP data modules. In the next section, we provide a table (Table 1) of the risk signals, which and in the ’As-Is’ situation are not presented to the approver in a consolidated, contextual way.
Table 1: Supplier Performance Data: Global Bio-Reagents Inc.
Metric | Data Point | Observation/ Trend |
Supplier De- livery Performance | On-Time-In-Full (OTIF) rate has fallen from 95% to 70% over the last 6 months. | A consistent and sharp negative trend, indicating deteriorating reliability. |
Quality Management Data | 3 new Quality Notifications (QNs #80012, #80015, #80019) in the last quarter. | A recurring GxP compliance issue specifically related to temperature deviations for “Stabilizer-7.” |
Goods Receipt Data | Batch acceptance rate at goods receipt has dropped from 100% to 92% in the last 2 months. | Increasing number of shipments failing initial quality checks, suggesting a potential systemic issue. |
Outcome of the ’As-Is’ Process: Missing a unified, con-textual view of these deteriorating trends, the procurement manager approves the PO. The shipment from Global Bio- Reagents arrives two days late. More critically, it arrives with another temperature excursion. This requires a mandatory quality investigation and corrective and preventive action (CAPA) plan, which takes time. The two-week delay and the investigation delayed and complicated the production of a vital Gerocept batch. This had an estimated opportunity cost of lost sales of US $1.2 million, plus an estimated $50,000 in internal costs to manage the CAPA. This event also created a high compliance risk that may be flagged in a global regulatory audit in the future.
4.2. The ’To-Be’ Process: AI-Powered Risk Mitigation
Now, think about the same PO #4500012345 being processed with the proposed AI-enabled framework which is intended to inject contextual intelligence into the approval workflow.
- PO Submission & Event Trigger: The PO is sent for approval in SAP S/4HANA. A Business Add-In (BAdI) developed in the Flexible Workflow sends a “POAp- provalInsightsRequired” event to the SAP Event Mesh. The event-driven model supports sidecar architecture and enables independence of the core SAP process.
- AI Insight Generation: A microservice on the SAP Business Technology Platform (BTP) listens for the event. When the assistant receives this notification, it collects all historical delivery, quality, and goods receipt information from S/4HANA using pre-defined Core Data Services (CDS) This context is then forwarded to a secure AI engine for a risk assessment and trend analysis to be carried out
- Actionable Insights Provided: The approver opens SAP Fiori’s ’My Inbox’ and selects the PO approval task. The approver sees more than just the basic PO data, they can engage with the AI generated summary located on the approval screen (as shown in Table 2). Now the approval screen is being used as an active decision point.
Table 2: Simulated AI Insights in Fiori App
Insight Component | Details & Justification |
Overall Confidence Score | 35% (High Risk) |
Key Risk Factor: Delivery Trend | Supplier’s on-time delivery for this material has degraded by 25% over the last six months, indicating a high probability of a schedule-impacting delay. |
Key Risk Factor: Quality Lapses | Recurring GxP compliance issues (3 QNs) for this specific material due to temperature deviations. High risk of repeat quality failure. |
AI Recommendation | Action: Reject PO. The combination of worsening delivery and repeated quality failures presents a significant and immediate risk to manufacturing continuity and compliance. |
Suggested Next Steps | Initiate an expedited order with the qualified secondary supplier and trigger a formal performance review for Global Bio-Reagents Inc. |
Quantified Benefits: The AI-based intervention has transformed approval from a procedural task to a strategic risk- reduced approval stage. By preventing the high-risk purchase order (PO), Innovida Life Sciences:
- Avoids $1.2 million in lost revenue as the Gerocept batch is able to be on time for production and avoid disruption to existing production operations.
- Avoids the $50,000 cost of the CAPA
- Obtain improved supply chain resiliency by correcting a weak-link in its supply chain which will generate systemic improvements in performance and patient
This pseudo case study has indicated that as a result of integrating contextual relevant AI-based intelligence, directly into the approving users workflow in the SAP PO module to transform reactive problem solving into proactive risk management, organizations have created value, both meaningfully and quantifiably.
4.3. Implementation Considerations: Cost-Benefit Analysis and ROI
While an accurate return on investment (ROI) is reliant on organization-specific elements, we can estimate a directional cost-benefit analysis that is rooted in the real-world application of the “Innovida Life Sciences” case study. This is also critical to make a business case for the upfront investment necessary for this kind of strategic implementation [2, 14].
Estimated Costs The implementation costs are a composite of technology, development, and ongoing maintenance.
- Technology & Infrastructure Costs:
- SAP BTP Services: The subscription costs associated with the main BTP services (SAP Event Mesh for the event-driven architecture, Cloud Foundry, or Kyma runtime environment for the microservice, SAP AI Core to manage the AI/ML model life cycle).
- AI/LLM Services: A large window for operational costs. Organizations can either use the Commercial pay-per-use Large Language Model (LLM) API and incur usage costs each time the LLM is used, or take the hit on the upfront investment to host a private company-native LLM that is similar to a LLM API but provides data security and privacy.
- Development & Implementation Costs:
- Personnel: One-time project cost of engaging a number of skilled people, including SAP BTP/ABAP developers to create and plan the integration, AI/ML engineering resources to develop prompt engineering, and model tuning.
- Data Integration: A substantial amount of working to develop and validate the Core Data Services (CDS) views to extract clean, reliable data from source systems (ERP, QMS, LIMS).
- Ongoing Maintenance & Governance:
- Model Monitoring: Considerable ongoing effort is required to monitor the performance of the LLM-enabled AI model to ensure the model is not drifting or ’hallucinating’, and to help maintain
- Change Management & Training: The upfront costs associated with training end users as well as the ongoing costs associated with managing the organizational shift to AI-assisted decision making are of considerable size, and in consideration of the importance of actually adopting the systems, have program affect changes in the organization too.
Projected Benefits & Value Proposition The benefits that are offered by the proposed solution go beyond straightforward automation and will provide considerable financial and strategic value too.
- Quantifiable (Direct) Benefits:
- Avoidance of Disruption Costs: As we illustrated in the case study, proactively avoiding just one high-risk PO can avoid major losses. The $1.2 million in lost revenue was avoided by stop- ping one production from stopping; this is a compelling rationale.
- Reduction in Compliance & Quality Costs: The framework will allow organizations to avoid direct costs due to quality failures, similar to the estimated $50,000 to investigate a CAPA, cited in the case study. As well, the level of manual work necessary for quality reviews will also be decreased.
- Increased Operational Efficiency: By automating the data gathering and analysis that an expert approver would do manually, the workflow system could shorten approval cycle time and release procurement professionals to spend more time on negotiations and on managing and developing suppliers.
- Qualitative (Strategic) Benefits:
- Enhanced Supply Chain Resilience: The value in moving from a reactive risk management approach to a proactive approach, will ultimately drive a more resilient and agile supply chain.
- Strengthened GxP Compliance: The system provides an automated, auditable control point in order to enforce GxP upstream in the procurement process, to protect product quality and patient safety.
5. Limitations of the Model Proposed
This model has considerable promise for improving supply chain management across the Life Sciences Value Chain, but it is also important to highlight the limitations and challenges of implementation.
5.1. Dependency on Data
The effectiveness of the AI analysis is dependent not only on the volume and granularity of data from potentially many data sources in SAP S/4HANA and other systems, but also on the availability, accessibility and quality of that data. Achieving a holistic view of a product’s lifecycle often challenges data silos and the need for quality data (which must be done with data integrity because of GxP) and data governance. In particular, the approach requires the integration of diverse systems such as the ERP, Quality Management Systems (QMS), Laboratory Information Management Systems (LIMS) and Manufacturing Execution Systems (MES), with effective data pipelines and validated data lineage that allows for an audit trail for everything the AI model ingests. Establishing this level of data integrity is substantial governance and technical challenge in and of itself.
5.2. AI Model Limitations
- Explainability and Trust: The “black box” aspect of some complex AI models can be a serious obstacle. To realize the complete transparency and auditability required for AI-generated risk scores within a GxP-regulated framework will need additional enhancements in the development and incorporation of Explainable AI (XAI) techniques.
- Bias and Accuracy: AI models can perpetuate biases in their training data, which, if not carefully addressed, can result in an unintentional and unfair supplier evaluation. In addition to addressing the uncertainty of the AI’s output, and preventing model drift, or “hallucinations”, will require continuous monitoring, and validation processes.
5.3. Implementation and Governance Risks
- Data Privacy within a Proprietary Context: Managing sensitive supplier data happens within proprietary The use of public AI services inevitably exposes this sensitive data to some risk. Therefore, this architecture proposes a strong recommendation for a proprietary or company-native LLM hosted in a secure setting to keep data confidential. All data transmissions must be encrypted, and access to the underlying data sources must be strictly governed based on roles [23].
- Regulatory Validation with a GxP Context: Within the Life Sciences industry, any system that can impact/affect quality decisions must undergo Computer Systems Validation (CSV) as part of any framework such as GAMP 5. This will require comprehensive documentation and auditability of the AI Model and decision-making logic [24]. For the AI recommendations to be validated, it will be crucial to be able to trace recommendations back to the physical data points that affect each recommendation because of the requirement for data lineage and integrity to prove GxP Changing the model would require a change control process.
- Complexity of Change Management: Implementing this system involves more than a technology change; it will involve changing core business processes, and possibly causing a change in the roles of people working with the system. Within the highly regulated, risk-averse atmosphere of the pharmaceutical sector, dealing with user adoption will be a significant road- block. Apart from user adoption, one of the greatest risks faced, will be user resistance to AI recommendations. Resistance needs to be considered in the context that trust must be established between the user and the AI To construct this level of trust, a change management strategy must promote the AI technology as a decision support system instead of a substitute for the decision being made [3, 16].
5.4. Technical Challenge and Cost
The implementation of this proposed event-based architecture, which will require technical use development, deployment, and ongoing stewardship, involves many technical challenges and associated expenses. Additionally, to ensure there is no excessive delay introduced into the time-sensitive PO approval process, the performance of the system must be managed carefully.
6. Future Opportunities
Can AI-based approaches to PO approval evolve and this framework for PO approval enhanced? The answer is yes with many possibilities for development and research to further build intelligent automation in Life Sciences SCLs.
Short-Term: Prototyping and Pilot Testing The next step in this project is the development of a prototype or pilot program in a controlled Life Sciences environment. In this environment, it will be possible to assess technical feasibility, refine the AI models and prompt, assess the usability of the interface, and collect empirical data on the systemic impacts the framework has on approval times, risk mitigation, and compliance adherence. Establishing robust methods and KPI’s to quantify and measure the ROI, and other tangible benefits gained using the intelligent approval system is also an important task. This aligns with the broader academic push to move from conceptual frameworks for AI in the pharmaceutical supply chain towards validated, real-world applications [25].
Mid-Term: Building on The AI Core and Explainability In future versions it would be possible to have access to a wider range of internal and external data sources (i.e. broader market intelligence, feeds on geopolitical risk, sustainability data, even more detailed IoT sensor data based on logistics/manufacturing) that would lend themselves to richer contextual analysis [4]. Additional investment in Explainable AI (XAI) methods inside the decision support interface, will be important for user buy-in and to facilitate regulatory audit requirements [26, 27]. There are many developing robust XAI capabilities, as an ongoing area of research, and which can make decision-making more effective in complex contexts such as SCL [13]. Indeed, there are views from other scientific disciplines regarding the challenges associated with the process of delivering trust in XAI methods in relation to dealing with complexity [13, 28]. Furthermore, designing into the AI system, the ability for the AI to learn from the outcomes associated with approved POs and any feedback from users on its recommendations, will enhance its predictive accuracy over time.
Long-Term: Strategic Deployment and Governance With the fundamental principles of AI contextual intelligence, it would be possible to expand and apply the concepts to other high-importance decision points with the SCM area of the SAP ecosystem, (e.g. supplier qualification and on-boarding, contract life cycle management, proactive quality event management). In deploying these concepts it will be important to have in mind the ongoing development and implementation of strong ethical guidelines, oversight frameworks, and management processes for AI execution in high-importance decisions in SCL, underpinned by the concepts of fairness, accountability, transparency, and bias mitigation [23, 24, 29].
7. Conclusion
The development of Purchase Order approval processes in SAP systems, moving from typical rule-based decisions to the more advanced, intelligent, contextually aware decision support systems discussed in this paper represents an important technological and strategic change that is necessary for modern Life Sciences supply chains. By combining the principles of Business Process Automation with the advanced analytical capabilities of AI and Machine Learning, especially when we can use this technology to embed con- textual intelligence and trend analysis into critical upstream controls like Purchase Order approvals, we can advance supply chain standards well beyond that of daily operational execution.
This shift in thinking represents more than just automating processes—it is about giving supply chain professionals advanced insight to gain insight into timely actionable in- formation at critical decision points in order to make better strategic decisions, to proactively address supply chain risks in relation to supply chain performance and quality, to adequately demonstrate GxP compliance, and ultimately to develop more robust and flexible Life Sciences supply chains. As intelligent technologies continue to evolve and be integrated across the SAP landscape (connecting the ERP with QM, IBP, MES, and outside partner data sources on networks), we can imagine a world where adaptive, predictive, and intelligent supply chain operations exist. This revolutionizes the way in which Life Sciences organizations can navigate the complexities of their global supply chain networks to manage reliable, compliant, and efficient material flows that are most critical for patient safety, regulatory compliance and sustainability in innovation.
- J. Y. Ma, L. Shi, T.W. Kang, “The effect of digital transformation on the pharmaceutical sustainable supply chain performance: The mediating role of information sharing and traceability using structural equation modeling”, Sustainability 2023, Vol. 15, Page 649, vol. 15, p. 649, 2022, doi:10.3390/SU15010649.
- R. Toorajipour, V. Sohrabpour, A. Nazarpour, P. Oghazi, M. Fischl, “Artificial intelligence in supply chain management: A systematic literature review”, Journal of Business Research, vol. 122, pp. 502–517, 2021, doi:10.1016/J.JBUSRES.2020.09.009.
- J. Idogawa, F. S. Bizarrias, R. Câmara, “Critical success factors for change management in business process management”, Business Process Management Journal, vol. 29, pp. 2009–2033, 2023, doi:10.1108/BPMJ-11-2022-0625.
- A. H. Ordibazar, O. K. Hussain, R. K. Chakrabortty, E. Irannezhad, M. Saberi, “Artificial intelligence applications for supply chain risk management considering interconnectivity, external events exposures and transparency: a systematic literature review”, Modern Supply Chain Research and Applications, 2025, doi:10.1108/MSCRA-10-2024-0041.
- J. Vellanki, “Discuss how ai-driven solutions can improve compliance with good practice (gxp) regulations in the biotechnology industry.-a comparative study”, International Journal of Research in Management Studies, 2024.
- A. Daios, N. Kladovasilakis, A. Kelemis, I. Kostavelis, “Ai applications in supply chain management: A survey”, Applied Sciences 2025, Vol. 15, Page 2775, vol. 15, p. 2775, 2025, doi:10.3390/APP15052775.
- A. Vaid, C. Sharma, “Pioneering digital transformation initiatives with cutting-edge sap s/4hana solutions”, World Journal of Advanced Engineering Technology and Sciences, vol. 3, pp. 109–121, 2021, doi:10.30574/wjaets.2021.3.2.0075.
- G. Baryannis, S. Validi, S. Dani, G. Antoniou, “Supply chain risk management and artificial intelligence: state of the art and future research directions”, International journal of production research, vol. 57, no. 7, pp. 2179–2202, 2019.
- S. Mundhra, K. S. Kumar, T. Prashant, “Harnessing ai and machine learning in pharmaceutical quality assurance”, Journal of Pharmaceutical Quality Assurance and Quality Control, vol. 6, pp. 19–29, 2024.
- A. K. Percherla, “Effortless logistics automation – sap logistics process automation with uipath rpa”, Journal of Artificial Intelligence & Cloud Computing, pp. 1–4, 2024, doi:10.47363/JAICC/2024(3)262.
- M. R. Kunchala, “Sap finance and management accounting with integration of ai and ml”, International Journal of Innovative Research in Engineering & Multidisciplinary Physical Sciences, vol. 10, no. 3, pp. 1–5, 2022, doi:10.37082/IJIRMPS.v10.i3.231962.
- M. Brylowski, M. Schroeder, S. Lodemann, W. Kersten, “Machine learning in supply chain management: A scoping review”, “Hamburg International Conference of Logistics (HICL) 2021”, pp. 377–406, epubli, 2021.
- F. Olan, K. Spanaki, W. Ahmed, G. Zhao, “Enabling explainable artificial intelligence capabilities in supply chain decision support making”, Production Planning and Control, vol. 36, pp. 808–819, 2025, doi:10.1080/09537287.2024.2313514.
- M. S. Attiany, S. A. Al-Kharabsheh, L. S. Al-Makhariz, M. A. Abed-Qader, S. I. S. Al-Hawary, A. A. Mohammad, A. A. A. Rahamneh, “Barriers to adopt industry 4.0 in supply chains using interpretive structural modeling”, Uncertain Supply Chain Management, vol. 11, pp. 299–306, 2023, doi:10.5267/J.USCM.2022.9.013.
- Y. A. Bena, R. Ibrahim, J. Mahmood, “Current challenges of big data quality management in big data governance: A literature review”, Lecture Notes on Data Engineering and Communications Technologies, vol. 210, pp. 160–172, 2024, doi:10.1007/978-3-031-59711-4_15.
- C. Kewalramani, S. Neema, “Generative ai in change management: A new framework for organizational transformation”, International Journal of Research and Analytical Reviews, p. 838, 2024.
- D. K. Vaka, “Procurement 4.0: Leveraging technology for transformative processes”, Journal of Scientific and Engineering Research, vol. 11, no. 3, pp. 278–282, 2024.
- C. M. Sakala, S. M. Bwalya, “The role of artificial intelligence in optimizing supply chain performance”, Journal of Procurement and Supply Chain Management, vol. 2, no. 1, p. 1–14, 2023.
- N. Y. Hussain, P. A. Adepoju, A. I. Afolabi, B. Austin-Gabriel, “Ai and predictive modeling for pharmaceutical supply chain optimization and market analysis”, International Journal Of Engineering Research And Development, vol. 20, pp. 191–197, 2024.
- J. Singh, “Event-driven architecture for real-time analytics in cloud crm platforms”, European Journal of Computer Science and Information Technology, vol. 13, pp. 24–34, 2025, doi:10.37745/ejcsit.2013/vol13n422434.
- M. C. Nezianya, A. O. Adebayo, P. Ezeliora, “A critical review of machine learning applications in supply chain risk management”, World Journal of Advanced Research and Reviews, vol. 23, pp. 1554–1567, 2024, doi:10.30574/WJARR.2024.23.3.2760.
- N. Bhatt, “Ai and big data analytics in pharmaceutical supply chain management”, International Journal of Scientific Research & Engineering Trends, pp. 599–601, 2023.
- T. Birkstedt, M. Minkkinen, A. Tandon, M. Mäntymäki, “Ai governance: themes, knowledge gaps and future agendas”, Internet Research, vol. 33, no. 7, pp. 133–167, 2023, doi:10.1108/INTR-01-2022-0042.
- M. Sendak, M. C. Elish, M. Gao, J. Futoma, W. Ratliff, M. Nichols, A. Bedoya, S. Balu, C. O’Brien, “”the human body is a black box”: supporting clinical decision-making with deep learning”, “Proceedings of the 2020 Conference on Fairness, Accountability, and Transparency”, FAT* ’20, p. 99–109, Association for Computing Machinery, New York, NY, USA, 2020, doi:10.1145/3351095.3372827.
- A. D. Adekola, S. A. Dada, “Optimizing pharmaceutical supply chain management through ai-driven predictive analytics: A conceptual framework”, Computer Science & IT Research Journal, vol. 5, pp. 2580–2593, 2024, doi:10.51594/csitrj.v5i11.1709.
- O. . Shaughnessy, P. Couto, M. Saarela, V. Podgorelec, “Recent applications of explainable ai (xai): A systematic literature review”, Applied Sciences 2024, Vol. 14, Page 8884, vol. 14, p. 8884, 2024, doi:10.3390/APP14198884.
- V. Hassija, V. Chamola, A. Mahapatra, A. Singal, D. Goel, K. Huang, S. Scardapane, I. Spinelli, M. Mahmud, A. Hussain, “Interpreting black-box models: A review on explainable artificial intelligence”, Cognitive Computation, vol. 16, pp. 45–74, 2024, doi:10.1007/s12559-023-10179-8.
- R. J. O’Loughlin, D. Li, R. Neale, T. A. O’Brien, “Moving beyond post hoc explainable artificial intelligence: a perspective paper on lessons learned from dynamical climate modeling”, Geoscientific Model Development, vol. 18, no. 3, pp. 787–802, 2025, doi:10.5194/gmd-18-787-2025.
- D. B. Patel, C. Author, “Ethical ai: Addressing bias and fairness in machine learning models for decision-making”, Journal of Computer Science and Technology Studies, vol. 3, pp. 13–17, 2021, doi:10.32996/JCSTS.2021.3.1.3.
- Vinil Apelagunta, Vishnuvardhan Reddy Tatavandla, “A Vendor-Agnostic Multi-Cloud Integration Framework Using Boomi and SAP BTP”, Journal of Engineering Research and Sciences, vol. 4, no. 12, pp. 1–14, 2025. doi: 10.55708/js0412001
- Vinil Apelagunta, Vishnuvardhan Reddy Tatavandla, “Implementing SAP Fiori in S/4HANA Transitions: Key Guidelines, Challenges, Strategic Implications, AI Integration Recommendations”, Journal of Engineering Research and Sciences, vol. 4, no. 11, pp. 1–9, 2025. doi: 10.55708/js0411001
- Vinil Apelagunta, Vishnuvardhan Reddy Tatavandla, “Energy-Optimized Smart Transformers for Renewable-Rich Grids”, Journal of Engineering Research and Sciences, vol. 4, no. 10, pp. 21–28, 2025. doi: 10.55708/js0410003
- Vinil Apelagunta, Vishnuvardhan Reddy Tatavandla, “Cloud ERP vs. On-Premise QAD ERP: A Cost-Benefit Analysis for Mid-Sized Manufacturers”, Journal of Engineering Research and Sciences, vol. 4, no. 7, pp. 1–14, 2025. doi: 10.55708/js0407001
- Vinil Apelagunta, Vishnuvardhan Reddy Tatavandla, “AI-Driven Digital Transformation: Challenges and Opportunities”, Journal of Engineering Research and Sciences, vol. 4, no. 4, pp. 8–19, 2025. doi: 10.55708/js0404002

