An Elaborate Breakdown of the Essentials of Biogas Production
Journal of Engineering Research and Sciences, Volume 1, Issue 4, Page # 93-118, 2022; DOI: 10.55708/js0104013
Keywords: Biogas, Biodigester, Pretreatment, Anaerobic digestion, Feedstock type
(This article belongs to the Section Chemical Engineering (CHE))
Export Citations
Cite
Abubakar, A. M. , Silas, K. and Aji, M. M. (2022). An Elaborate Breakdown of the Essentials of Biogas Production. Journal of Engineering Research and Sciences, 1(4), 93–118. https://doi.org/10.55708/js0104013
Abdulhalim Musa Abubakar, Kiman Silas and Mohammed Modu Aji. "An Elaborate Breakdown of the Essentials of Biogas Production." Journal of Engineering Research and Sciences 1, no. 4 (April 2022): 93–118. https://doi.org/10.55708/js0104013
A.M. Abubakar, K. Silas and M.M. Aji, "An Elaborate Breakdown of the Essentials of Biogas Production," Journal of Engineering Research and Sciences, vol. 1, no. 4, pp. 93–118, Apr. 2022, doi: 10.55708/js0104013.
World search for ways to properly manage rural and urban waste generated on daily basis from domestic and industrial buildings, perhaps leads to the adoption of the anaerobic digestion (AD) systems. The system utilizes microorganisms such as viruses, fungi, helminths, bacteria and protozoa to degrade waste so as to generate useful by-products such as biogas, used in heating, lighting and as fuel. Research on ways to effectively generate biogas from different feedstock had been serious in recent years, especially the study of the process kinetics to maximize production. This review seeks to provide details on feedstock type, pretreatment, substrate degradation, biogas properties, biogas utilization and factors influencing its production. Conclusion is drawn, noting that maximum biogas yield can only be obtained if the production parameters are carefully selected. Pressure being among the factors affecting the microclimate of digesters, is often uncontrolled in most biogas production facilities being slightly above the atmospheric pressure. Recent findings shows that biogas/methane amounts is increased with decreased internal gas pressure and could be a new efficient and effective process control strategy together with pH and temperature. This work will equip researchers and biogas plant developers with the rudiments of the technology which will eliminate the lack of technological know-how often experienced in some realms; in order to breach the gap in production and create a balance between waste generation, recycle and reuse.
1. Introduction
In the 17th century, Robert Boyle and Stephen Hale produced for the first time, biogas from decaying organic matter [1]. Sir Humphry Davy identified methane in the gas generated from cattle manure decomposition in 1808; however, methane was first identified via experimentation in 1776 by Alessandro Volta [1–3]. Five decades later, at Bombay, India, the first anaerobic digester was built in 1859. Though China is presently leading in the development of the technology, it all began in 1920, when Luo Guorui built the first hydraulic digester called ‘Chinese Guorui Natural Gas Stove’. In 1930s, the discovery gained academic recognition leading to scientific research [1]. Germany’s main feedstock for biogas production in 1945 was agricultural products. With increasing awareness of the technology, the 1950s witnessed an upsurge in the development of biogas plants [2]. Early biogas plant builders in Africa are Algeria, followed by Kenya and Tanzania, between 1930s and 1950s. Up till this present time, more and more plants are been built as part of policy strategy of government of some countries and/or local and international organizations with aims including, poverty reduction, economic growth, electricity generation, improve agricultural yield, solution to pollution problems and to arrest the menace of desertification.
By 2050, a 70% rise in world waste generation, triggered by industrialization and population growth is predicted [2]. This will increase the demand for energy and fuel of which biogas is a good candidate. Currently, thousands of biogas plants are being in operation in Africa, North America, Europe and Asia [4–6]. Germany with 4000 biogas plants occupies the leading position in Europe in biogas production, majorly utilizing farm residue for coproduction [7]. Sweden has 233 biogas facilities while Austria in 2008, a report, puts the number of biogas plants for green electricity production at 294 [7]. Europe is the leading continent in biomethane production (2.4 billion m3), with 18,943 plants feeding 725 biomethane plants and producing 15.8 billion m3 of biogas [8]. Authors like [9], [10] and [11] gave insight on cooking preferences of biogas, potentials of livestock and agricultural waste for biogas production, and the energy potentials of biogas in their respective countries, which are India, Greece, China and Ukraine. In the Mekong Delta region of Vietnam, super-intensive shrimp aquaculture is becoming prominent and the AD of shrimp sludge with other biomass gives promising result [12]. Indonesia targets 16.9% increase in biogas exploitation for power generation by 2025 [13]. Other Asian countries have the following numbers of digesters: China, 20 million family size type; India, 100, 000 digesters; Korea, 24000; and Taiwan, 7,500 biofermenters [9–11]. In Africa, lack of technical know-how is one of the reasons that puts most of the biogas plants in the continent out of operation, especially in Zimbabwe, where this gases are been flared [14]. Egyptians use underground biodigesters; Ethiopia employs fixed dome bioreactor in about 4500 household utilizing kitchen waste; while it has been reported that biogas potential in Mauritania is 520-258.7 (±125.8) 106 m3/year [5, 15–17]. Guinea witnessed their first digester in Kindia and Macenta in 1977 and from 1981-1999, 90 more plants was installed, which died out as a result of non-monitoring [3]. But in 2016, 2000 digesters was reported by the same author to have been constructed by the government. East and North Africa could boost of 3.2 million m3/h of biogas generation according to [5, 15–18].
The larger the volume of useful organic waste generated by a country, the higher its potential for biogas production. In developing countries, 93% of waste generated are dumped at road-sides, open lands and waterways or burned/incinerated – but world over, almost 40% of waste goes to landfills [19]. Despite the fact that Nigeria, Africa’s most populous country, generates massive amounts of trash, the government have made little effort to develop a biogas plant to benefit from any of its products. However, various bench-scale biodigester investigations have been conducted in Nigerian polytechnics and universities solely for research purposes [18]. In addition, Canada’s waste generation is estimated to reach 35.5 million tonnes [8]. But the potential in Canada is below countries that can boost of huge number of industries generating degradable byproducts, large hectares of land yielding high agricultural residue, large animal population or whose human population has the potential of generating enormous municipal waste, such as China, India Indonesia, Nigeria, Brazil, United States and Germany.
Setting up a biogas plant to be operated for large scale production of biogas or a small size one for domestic use, all require expertise and adherence to conditions that suits the manufacturing process. Research is ongoing to find ways of achieving optimum production by combining certain constraint parameters of production. In essence, the versatility of the process is increasing due to new findings often reported. In 2010, Mohamed Samer, developed a software to facilitate the planning, design, dimensioning and estimating the amount of materials needed for biogas plant construction together with cost analysis capability [20]. In the internet, many experts have tried to replicate this using Excel Spreadsheet or through the development of a PC or android software (e.g. BiogasApp, India) that gives the biogas and energy output of some feedstock in their locality [21]. Existing softwares are Anessa AD.A used for biogas plant feasibility assessment and operation, SMART BIOGAS, SIMBA#Biogas for biogas plant simulation and the software for biogas research by S.D. Hafner (2018). Also, the study on biogas production using ASPEN by process engineers is scanty [22, 23]. In this work, the various type of digesters for anaerobic decomposition, the feedstock type, feedstock preparation, degradation stages, variables influencing production, biogas characteristics and cleaning are highlighted.
2. Biodigester
Bioreactors is also termed digesters – an airproof reactor tank or vessel that is simple, cheap, robust, easy to operate and maintain [24–26]. Biogas digesters or simply biodigesters are considered small-scale if subjected to domestic use and large-scale, as in industrial digesters [27]. They are flexible because they are made of plastics such as polyvinyl chloride (PVC) and low-density polyethylene (LDPE) or high-density polyethylene (HDPE) [28]. Microorganisms such as fungi, bacteria and protozoa can survive in an oxygen-void environment, degrading the feedstock to produce biogas and digestate. The process is identical to what happens in a cow’s stomach, where stomach bacteria convert food into dungs and biogas as shown in Figure 1 (a mixture of methane and carbon dioxide gas) [29].
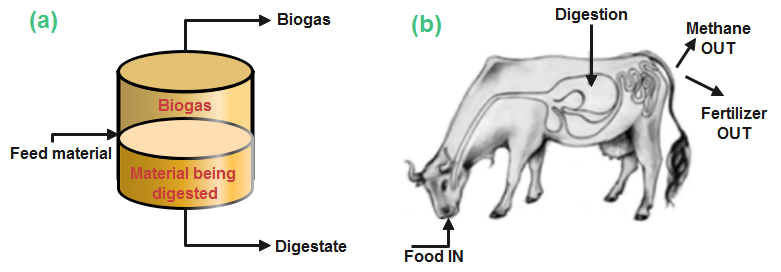
Not only methane as shown in Figure 1b is produced, but several other gases constitute the biogas end-product. The more the waste is degraded, the more the gas is produced [30]. The decomposed substrate is the residue called the digestate which is rich in macro- and micro nutrients, and used as biofertilizer [25, 31, 32]. The digestate will have little or no smell if the digester is working perfectly [29].
2.1 Biogas Composition and Properties
Biogas is a colorless and odourless gas composed of methane (CH4), carbon dioxide (CO2), hydrogen (H2), hydrogen sulphide (H2S), ammonia (NH3), nitrogen (N2), oxygen (O2) and water vapor (H2O) in varying proportions. Main compound present is CH4; in some cases reaching up to two-third of the whole composition, and referred to as the energy component of the biogas [21, 33, 34]. Second most-highest concentration in biogas is CO2, which could diminish it’s energy value [6]. Hydrogen sulphide (H2S) is noxious and toxic when in high concentration in biogas, whereas siloxanes: a silicon derivative gotten from decomposition of cosmetics is rarely present [33, 35]. Table 1 shows typical biogas compositions reported from biogas analyzers such as Gas Chromatography (GC), Fourier Transforms Infrared (FTIR) spectroscopy and Gas Chromatography-Mass Spectrometry (GC-MS), Gas Chromatograph coupled with a Thermal Conductivity Detector (GC-TCD) among others [36].
Table 1: Typical Biogas Composition [4, 15, 21, 24, 25], [33–35, 37–41]
Component | Percentage (%) |
CH4 | 35-75 |
CO2 | 15-65 |
H2 | 0-7 |
H2S | 0-3 |
NH3 | 0-2 |
N2 | 0-10 |
O2 | 0-2 |
H2O | 1-7 |
Trace gases | < 2 |
Despite the multitude of gaseous compounds present in biogas, it is still 20% lighter than air [14, 42]. Yield and composition of biogas depends on digestion condition, feedstock and co-substrate type [15, 34]. Biomethane are upgraded biogas where composition of more than 40% CH4 will be responsible for the characteristic flammability of the gas [29, 43, 44]. This flame is hotter than fire and is clear, similar to liquefied petroleum gas (LPG) blue [15, 42, 45, 46]. Biogas are characterized with low energy density, slow flame speed and partial combustion; a property that is considered negative [34]. In addition, ignition temperature is in the range of 650-7500C [14, 42]. Gaseous compounds including NH3, H2S and CO2 in biogas are poisonous, which is the main reason biogas can suffocate anyone exposed to it in an enclosed area [29]. Removal or reduction of these poisonous gas, brings biogas to the level of natural gas or fuel grade CH4, especially using the pressure swing adsorption (PSA) technique, as reported by [47]. For instance, CO2 content could be brought to desirable level during upgrading process by scrubbing as well as the use of recent technology known as the microbial electrochemical to reduce CO2 [48]. [49] stated that biogas recirculation is cost-effective when it comes to enhancing the quality of biogas in AD reactors while [50] explains biogas up-gradation into syngas via dry reforming. Mega joules (MJ) is the energy units of biogas and 1 m3 of raw biogas at STP containing 60% CH4 will give a heating value of 21.5 MJ (5.97 kW h) [27, 34].
Dangers faced in the use of biogas comes from the CH4 content and is the reason for the hazardous characteristic of biogas. The safety measures to be taken while operating biogas plants on either large-scale, small or household size basis are the wearing of protective equipment, avoidance of contact with digester content through the use of gloves and regular hand washing [11, 29]. Electric spark triggering explosion, fire when naked flame is brought closer to gas or through smoking, risk of diseases such as malaria, cholera and typhoid especially while handling livestock waste, H2S poisoning (up to 10-150ppm causing lungs irritation), smell when digester is dysfunctional and asphyxiation due to inadequate ventilation are potential dangers associated with biogas production [17, 29, 33, 51]. Threshold percentage resulting in explosion is observed in two cases; when 10-30% of the gas is diluted with air and when the biogas containing 60% of CH4 mixes with air [51]. The same author reported that, even though CH4 is non-toxic, concentrations between 5-15% is explosive.
2.2. Categories of Feedstock for Anaerobic Digestion (AD)
Organic wastes are the main feedstock for AD and is divided into three broad categories including agricultural, municipal and industrial waste [4, 43, 52]. Agricultural wastes are livestock residue, garden waste, harvest residue, energy crops, vegetable by-products, grasses (e.g. steamed lemon grass and sudan grass) and algae (e.g. Spirogyra neglecta and Cladophora glomerata) [12, 18]. In [53], it was reported that 1 kg of water hyacinth will generate approximately 0.014 m3 of biogas. Livestock residues are obtained from slaughter houses, ranges, pisciculture, insect farms and poultry houses, which are fish residue (e.g. shrimp sludge, fish meal, fish maw and isinglass), insects and worms, poultry litter, keratin-rich waste and manures [12, 39]. 1 kg of cattle dung, pig dung, chicken droppings and chicken manure will generate approximately 0.04 m3, 0.06 m3, 0.07 m3 and 0.065-0.116 m3 of biogas respectively from 1:1 water-to-feedstock ratio [53, 54]. Volume of 1 kg of fresh cattle dung is about 0.9 litres, containing 8% dry biodegradable mass. Over 250 million cattle population in India is a promising potential for biogas and energy generation [7]. If manures are not properly handled, they can result in emission of nitrous oxide (N2O) to the atmosphere, whose negative effect on the climate is 265-298 times greater than CO2, contributing to about 10% of global non-CO2 emissions [55, 56].
Insect farming technology breeding silkworm and caterpillar excreta generates biogas comparable to animal waste; specifically silkworm excreta was reported to generate 331.97 m3/Mg TS [57]. Poultry litter are lignocellulosic bedding materials containing wasted water, spilled feed (e.g. grains, peanut hulls and pine straw), wood shavings, poultry manure, feathers and sawdust [58, 59]. Keratin-rich waste are fibrous protein in the form of skin, wool, chicken feather, horns, mixture of borns, hooves, beaks, hair, nails, organs, hard tissues and claw produced by fish, meat and wool industry, in large quantity [60]. Manures are animal droppings which is a mixture of H2O, straw, excreta (faeces and urine), livestock bedding, sand and wasted feed, that is rich in NPK and fiber; and is obtained from elephant, cattle, sheep, goat, chicken, camel, donkey, pig, rabbit, deer, horses and duck [48, 49, 61, 62]. Manures can be used to produce biogas, biofuel and synthetic gas – while on the other hand, manure fibers can be used to produce building materials, plant growth medium identical to peat moss, paper, seed starter pots and fertilizer garden sculptures, thereby changing its environmental liability status to a useful product. Carbohydrate, fat, protein, crude fiber and ash contents in pig, cow and chicken manures are respectively, “38, 4, 19, 20 & 19%”, “20, 4, 15, 40 & 21%” and “25, 4, 29, 15 & 27%”, according to [7]. Taiwan have constructed over 7,500 CH4-generating devices, that utilizes pig manure in Taiwan while Poland produces 112 million Mg of manures yearly making it the largest producer in Europe, but utilizes < 1% to manufacture biogas [56].
Municipal wastes are divided into municipal solid waste (MSW) and liquid waste, including food waste (FW), municipal waste water, landfill waste, papers, green waste, urban sanitation and aquatic biomass, gotten from diverse sources such as domestic, educational, industrial and medical facilities [8, 63, 64]. Annually, 2 billion tons of MSW is generated worldwide, which is projected to rise to 3.4 billion tons by 2050 [19]. FW is defined as a variable substrate, uneaten, discarded or lost during stages of production, processing, distribution and consumption of foods including rice, yam, noodles, nuts, pasta, eggs, fish, bagasse, vegetables, oil waste, fruits, meat, potato, and sweets [65–70]. Huge amounts of FW are products from restaurants, canteens, markets, hotels, hostels, food processing industries and households coming from kitchen’s of the listed buildings and locations [34, 60, 71]. Dirty water and remains of different food types are generated in the kitchen, and are for example, tuber peels (e.g. potato peels), vegetable residue, fruits peel, cooked food leftovers and spices [71, 72]. Spices including red chili, black pepper, cinnamon, coriander, garlic, turmeric, cardamom and clove are unsuitable for AD process [73]. Waste oils such as cooking oil, essential oils, microalgal oil, fish waste oil, fat, grease and palm oil are feedstock for biogas production [74]. Presently, Indonesia remains the largest palm oil producer in the world and more production will balance the gap between increasing demand for the product (rose by 186% from 2010-2025) [75]. Municipal waste water like sewage sludge and water from gutters are typified by low recovery of biogas [45, 76, 77]. In developing nations where there are no good drainage systems, the waste waters cannot be easily tapped and harnessed. Potentials of human faeces for biogas production have also been experimented [13]. Depending on the climate, diet, food and water intake, biogas production rate of human excreta is 0.02-0.07 m3/kg day, while daily production of this waste from an average adult human is 1-1.3 kg of urine and 0.2-0.4 kg of faeces as reported by [54].
Landfill site receives almost all types of waste, grouped into organic and inorganic sub-types of which typical example is MSW disposed at landfill via composting or open dumping and leachate formed at landfill sites which yields considerable percentage of biogas during AD [4, 45, 63, 78]. A linear statistical model had been developed by [79] to model the energy potential of the MSW comprising of woods, grasses, papers, leaves, food remnants, plastics, metals and glasses in nine densely populated Northern states in Nigeria to serve as a tool for setting up energy policy that will aid waste management in the region. Waste recovery in one of the state (Maiduguri), such as metals, plastics, bottles, ceramics, paper and magazines from waste collection points and dumpsites is an activity that would be responsible for poverty alleviation and job creation, especially when paper and magazines recovered are channeled to biogas generation [80]. Also, analysis of leachate samples in four dumpsites of the same location (Ajaganaram, Bulabulin, Gwange & Monday Market) is reported to be rich in metallic nutrients that would support AD if exploited by the locales [81]. Paper sludge, with potential of 14.7 mL/gVS of biogas, emanates from the paper mill primary clarifier in the water treatment unit of the industry [82]. Generally, papers contain two types of structural carbohydrate (cellulose and hemicellulose), which are ideal AD substrate found in paper and pulp industry, printing press and learning institutions, including cardboard, filter paper, waste paper, newspaper, tissue paper and magazine [83, 84].
Aquatic biomass are possibly aquatic weeds such as floating weeds (e.g. water hyacinth or Eichhornia crassipes, Azolla pinnata, duckweeds or Lemna perpusilla, Pistia stratiotes, Neptunia oleracea, Pandanus helicopus, etc), emergent weeds (e.g. Nymphae spp., Nelumbo nucifera, Myriophyllum aquaticum, etc), algal weeds (e.g. Microcystis aeuginosa, Dinoflagellates spp., Oscillatoria, etc), marginal weeds (e.g. Marsilia mutica, Typha angustifolia, Colocasia esculenta, Cyperus papyrus, etc), submerged weeds (e.g. Myriophyllum spicatum, Vallisneria spiralis, Ceretophyllum demersum, Hydrilla verticillata, chara/muskgrass or skunkweed, etc) and water primrose (Ludwigia hyssopifolia), which are rich lignocellosic biomass [18, 24, 66, 67, 85, 86]. Invasive aquatic weeds are found in India, Indonesia, Australia, Brazil, North America, New Zealand, Central America, Malaysia, South-East Asia and Africa (places include Rivers of Southern Mozambique, South Africa, Kenya, Ghana, Mali, Egypt, Benin Republic, Volta River of Burkina Faso, Niger Republic, Sudan and Nigerian coastal creeks and lagoons); but it is most-abundant in Indonesia [85–93]. They are found in almost every part of the world and had been reported to be a good source of biogas generation with potential for power generation in Kenya [92]. In the literature, water hyacinth is the most used aquatic biomass for biogas production because it is abundant, having the capacity to double their number in just 2 weeks, constituting a pollution to water bodies [94, 95]. It originated in Brazil and was 1st seen in 1984 around Badagry Creek in Lagos; River Benue at Makurdi in 1988; and River Niger and Kainji Lake in 1992, all in Nigeria [89, 96]. In [97], the potentials of water hyacinth’s application for biogas synthesis by communities living near the Lake Chad Basin in North Eastern Nigeria had been highlighted. In addition, communities living near River Niger, River Kaduna, River Benue, Kainji Dam and the Kaduna River in Nigeria can also make use of the available biomass. Using conical flasks as digesters, [95] studied the possibility of reaping considerable volume of biogas from floating aquatic weeds. Other aquatic biomass are Elodea canadensis, considered as the most widespread plant in Europe; Azolla filiculoides ranks second followed by Vallisneria spiralis and Elodea nuttallii in the same continent; Typha angustifolia, whose infestation in the wetlands of Hadejia-Jama’are, Lake Chad Basin and Sokoto-Rima river basins in Northern Nigeria constitutes a major problem; and water fern (Salvinia molesta) which produces (on average) 6.7 l/kg of biogas from 1st ever recorded research carried out by Abbasi & Nipaney in 1984 [86, 88, 96, 98, 99].
Industries processing food and agricultural products, pharmaceutical industries, fodder and brewery industry, sugar industry, fruit processing, textile industry and wastewater treatment plants (WWTPs) are some of the industries generating semi-solid and liquid organic waste for anaerobic digesters [26, 68, 69, 100, 101]. WWTPs generate semi-solid waste by-products known as sludge, which is sub-divided into settled primary sludge or waste activated sludge (WAS) generated during biological treatment of which >18,000 of these plants are in Europe alone [31, 59, 70, 102]. Domestic and municipal wastewater produced worldwide is around 360,000 m3 yearly out of which 52% is mostly treated in WWTPs according to Utrecht University and the United Nations (UN)’s findings [103]. Two types of waste are gotten from fruit processing facilities which are solid waste consisting of stones, skin, seeds and peels, and liquid waste from juice and wash-waters [60, 104–109]. In [110], MSW (FW inclusive) is predicted to rise to about 72,146 tons/day in South Africa by 2025 as a result of population growth. Pharmaceutical wastewater is the least exploited source of biogas production compared to the textile and beverage industry effluents. Tetracycline antibiotic has been shown to enhance production in a lab-scale anaerobic baffled reactor (ABR), whereas herbal pharmaceutical wastewater could generate about 43.3% CH4 when digested [111, 112]. Cotton yarn wastes and textile wastes (of which globally, 75% is disposed of in landfills) could be reused or recycled back into clothing or channeled into bioenergy manufacture [113, 114]. This venture might not be easy as effluent from textile industries are characterized by toxicity, lower pH and carbon-to-nitrogen (C/N) ratio [115, 116]. The beverage industry has been given the desired concern by researchers, especially in the application of soft drink beverage waste, native beverage vinasse and alcoholic wastewater [100, 117, 118].
It is possible to distinguish two major types of waste that can be digested into liquid effluent waste (e.g. wastewater, manure slurry, sewage sludge, agro-food effluents, etc) and organic solid waste (e.g. agricultural, industrial and municipal waste) [33, 76]. It is advisable, most times, to opt for substrates looking at its sustainability, energy recovery, digestibility, yield potential, environmental and economic values [15, 18, 69].
2.3. Feedstock Preparation
Certain amount of inorganic contaminants (e.g. debris, grits, glass, sticks and metals) are advised to be removed prior to subjecting them to AD to produce biogas [15, 47]. Pretreatment, addition of additives, degradation stages and biogas storage and utilization must also be considered. Pretreatment breaks down the lignin layer and chemical properties of lignocellulosic matter, increasing its resistibility to degradation by enzymes and bacteria during AD [24, 60]. Untreated lignocellulosic raw matter are bulky and difficult to feed into conventional biogas digesters [119]. It is highly required to select appropriate pretreatment process for a sustainable conversion into bioenergy [120]. Table 2 shows the advantages of subjecting organic feedstock to pretreatment stages.
Table 2 Advantages of Substrate Pretreatment
S. No. | Merit of Pretreatment | Reference |
1. | It enhances degradation of substrates and increase process efficiency | [34] |
2. | Reason for sustainable conversion of feedstock into renewable energy source | [120] |
3. | Remove potential inhibitors in feedstock, decrease crystallinity of cellulose and increase its porosity | [60] |
4. | Lowering the degree of polymerization | [40] |
5. | Reduce the required retention time (RT) for AD | [69] |
6. | Accelerate hydrolysis | [121] |
7. | Increase the accessible surface area and consequently improve CH4 production | [31, 122] |
Ideally, pretreatment methods are grouped into physical, chemical, biological and combined methods [31, 34, 59, 84, 100, 123].
2.3.1. Physical Pretreatment
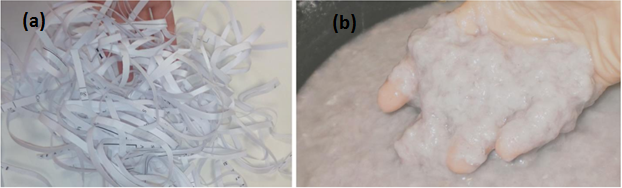
Alternatively, physical pretreatment is known as mechanical pretreatment. Since substrate particle sizes directly influence AD, the sole aim of carrying out physical pretreatment is to reduce the sizes of raw materials, thereby increasing the surface area for hydrolyzing enzymes, enhancement of heat and mass transfers and in knowing the viscosity of the slurry [15, 34, 124–126]. The technique is popular and involves the use of knives, blades and hammers to grind, chip, mill, crumble, cut and shred biomass into small particles before AD [6]. Mechanical pretreatment method is a technique applied to change the appearance or structure of the biomass by mechanical means including extrusion, comminution/milling (e.g. ball milling), steam explosion, liquid hot water pretreatment, microwave irradiation and ultrasonic treatment [47, 84, 122]. Figure 2 shows a shredded paper before and after pretreatment.
Extrusion involves the use of an extruder which subjects the lignocellulosic biomass to a series of treatments, such as sudden pressure drop, heating and mixing [127]. Thermal pretreatment involves the use of steam or hot water at 50–250°C to effect cell wall disintegration through the breaking of the hydrogen bonds that maintain mechanical strength of the biomass [128, 129]. This can further be sub-divided into low temperature (< 110°C) and high temperature (> 110 °C) reactions [6]. Another type of thermal pretreatment technique is high pressure steam explosion, required for disrupting the lignocellulosic structure of substrates [38]. It requires a very high pressure (5-60 bar) and temperature in the range of 160–250°C [127]. Liquid hot water pretreatment uses elevated temperature (140-220°C) and pressure to keep H2O in liquid state [130]. Also called hydrothermal or pressurized hot water pretreatment, with benefits such as reduction in the risk of inhibitors production (e.g. furfural), increase enzyme accessibility and the ability to efficiently solubilize hemicellulose and lignin [15, 16, 131]. Microwave irradiation is quick in disintegrating sludge, as well as ensuring its solubilization and degradability. It destroys the faecal coliforms and Salmonella spp. and also increase the hydrolysis rate of lignocellulosic matter, especially straw. Other physical pretreatment approaches for sludge disintegration such as pulse electric fields, grinding, high-pressure homogenization, microwave irradiation, lysis centrifuges and ultrasonication were highlighted by [31]. Microwave irradiation heating and ultrasonic pretreatment can transform the internal microstructure of straw [130] as depicted in Figure 3.

2.3.2. Chemical Pretreatment
Chemical pretreatment is based on the application of acid or base to improve raw material degradation rate as well as to break down the covalent bond of the lignocellulose [100, 130]. Acid treatment disrupts the Van der Waals, hydrogen and covalent bonds that bind the molecules of the organic matter before decomposition using either sulfuric (H2SO4), hydrochloric (HCl), formic, nitric acid (HNO3), phosphoric acid (H3PO4), hydrogen peroxide (H2O2) and/or ethanoic acid (CH3COOH) [38, 127, 132]. High-concentration acid pretreatment is done at low temperature whereas low-concentration pretreatment is carried out at high temperatures [68]. Alkali treatment method causes swelling of the fibers thereby conditioning the biomass to efficiently serve the AD process at different concentrations of lime (Ca(OH)2), sodium hydroxide (NaOH), sodium carbonate (Na2CO3), sodium bicarbonate (NaHCO3), aqueous ammonia (NH3∙H2O), potassium hydroxide (KOH), urea or NH3 solution by soaking or spraying on the raw material surface [24, 132–134]. Alkaline pretreatment with ozone (O3) (ozonolysis) and H2O2 has been reported to have the same effect with the forgone base compounds [31, 120, 132].
In general, chemical pretreatment are limited in application due to high cost of chemicals and pose environmental side effect [34, 59]. It is fact, that chemical pretreatment with alkali is better than acid pretreatment. Volume of acid/base, time and reaction temperature cannot be the same for all feedstock, as mass of feedstock taken for pretreatment is based on researcher’s discretion. For instance, [135] had tested 3 different chemical pretreatment techniques, namely organosolv, N-methyl morpholine N-oxide (NMMO) and alkaline pretreatment for 15g, 7.5g and 16g of wheat straw respectively, at different temperature, molarity of chemicals and reaction time.
2.3.3. Biological Pretreatment
Biological pretreatment entails applying micro-organisms (fungi, archaea, protozoa and bacteria) and enzymes to organic matter to break down lignin and hemicellulose [100, 136]. Growth and metabolism of anaerobic fungi (Piromyces spp., phylum Neocallimastigomycota, Saccharomyces cerevisiae, white and soft rot fungi) are crucial factors to their effective utilization [127]. White rot fungi is the most prominent microbe extracted from decaying wood that is used in biological pretreatment [6]. Source of Neocallimastigomycota are faeces and digestive tracts of various large mammal herbivores (e.g. elephants, goats, rhinoceros, horses, buffalos, cows, camels, and sheep) as well as reptiles and mice. Glycoside hydrolases, carbohydrate esterases and polysaccharide lyases are various enzymes used for this technique [136]. [137] also views the utilization of insect gut bacteria as a promising tool to enhance biogas generation.
Biological pretreatment is a slow process requiring longer RT, but beneficial, as it accelerates the hydrolysis process, has less energy consumption, long pretreatment cycle, has mild reaction conditions, needs efficient biological bacterium agent and cover large area [68, 130]. Application of biological pretreatments has been patronized in recent years because of the complex composition of lignocellulosic resources persistent in anaerobic environments, desire to reduce hydraulic retention times and the wish to increase the net carbon conversion rates [138]. Biological pretreatments can be divided into three parts including enzymatic, anaerobic and aerobic [70]. Biological pretreatment effect the necessary changes much better than physical and chemical pretreatment, but needs efficient bacterium agent [130]. Huge operational cost and high energy demands are the major disadvantages associated with physical, chemical and thermal pretreatments, which make those pretreatment technologies unsuitable and almost impractical [120].
2.3.4. Combined Pretreatment Method
Sometimes one substrate is subjected to two pretreatment methods simultaneously, implying a combined pretreatment procedure. Often times, a combination of physical and chemical methods are applied because they are faster and easier to implement [70, 84, 100]. Such combinations has synergistic pretreatment effect and functions better than single method given that an environmentally friendly pretreatment method is desired [121, 122]. But combinations sometimes, comes with unique disadvantages; for instance, chemical treatment has pollution consequences while biological treatment is challenging to monitor; but a combination of the two carries the two drawbacks together [139]. Pretreatment period beyond 3-30 days is not conducive according to [24]. Objectives, any disintegration/pretreatment technology is poised to achieve are basically, release of organic substrate (increase in COD solubilization), elimination of foaming in digestion chambers and secondary settling tanks, increase in the biogas yield, and access to trapped organic substances inside the biomass [121].
Composting could also be applied as a pretreatment technique prior to biogas production, but composting may lead to organic matter degradation and consequently reduced biogas yield during the AD process and so, partial composting has been suggested to avoid organic matter loss [140]. Major reasons why some feedstock are not ideal for biogas production is that they are difficult to digest by microorganisms, they exhibit slow digestion rate and are contaminated by inhibitors [60]. The goal of the pretreatment steps is to facilitate the digestion process by removing these barriers.
2.3.5. Additives
Certain adsorbents, enzymes or catalyst may be added to the bioreactor housing the feedstock for AD to optimize the yield of CH4 as well as stabilize the process. Pectin, silica gel, bentonite, tale powder, activated carbon, kaolin, gelatin and polyvinyl alcohol are examples of adsorbents that can work the talk [69]. Examples of biological catalysts are plants, crop residues, weeds, microbial culture, powdered leaves, legumes and cellulolytic strains of bacteria. Among these legumes are Acacia auriculiforms, Eucalyptus tereticonius, Gulmohar, Dalbergia sisoo and Leucacena leucocephala – capable of stimulating 18-40% increase in biogas production [141]. Production was also reported to grow up to 8.4-44% using cattle dung plus cellulolytic bacterial strains like actinomycetes [141]. Rumen fluids are rich in cellulolytic and methanogenic bacteria which are used as biostarters to shorten maximum biogas production time and increase production [87]. Enzymes are typically biocatalyst such as lyases, oxidoreductases, hydrolysases, transferases, ligases and isomerases for enhancing biomass disintegration biologically [121]. The most commonly explored catalyst are magnetite, silica gel, zeolite, and natural clinoptilolite [48]. In [142], guar gum’s ability to enhance biogas production from coal has been demonstrated.
2.4. Stages of Degradation
AD process consist of four stages, which are in the order of hydrolysis, acidogenesis, acetogenesis and methanogenesis, transitioned by various set of microbes [45, 59, 67, 73, 115, 129, 143–145]. The acidogenesis and acetogenesis stages are sometimes coupled together as the acidification step making it a three stage process of hydrolysis, acidification and methanogenesis [131]. Two stage AD involves two separate bioreactors for acidogenesis and methanogenesis [69, 128].
2.4.1. Hydrolysis
Hydrolysis is the first step as well as the rate-limiting step of the AD process [4, 16, 58, 129]. Here, insoluble complex organic hydrocarbon polymers (such as carbohydrates, lipids, polysaccharides, proteins and nucleic acids) are converted or depolymerized into simple, soluble, low molecular weight simple sugars or monosaccharides, long chain fatty acids, amino acids, purines and pyrimidines by hydrolytic bacteria (e.g. Clostridium, Micrococci, Bacteroides, Butyrivibrio, Fusobacterium, Selenomonas, and Streptococcus, among others) by secreting enzymes such as cellobiase, amylase, lipase, cellulose, xylanase and protease [34, 45, 49, 69]. In the process, cellulose is hydrolyzed to glucose while hemicellulose are decomposed to monosaccharides like xylose, glucose, galactose, arabinose and mannose [77]. If the feedstock contains carbohydrate, hydrolysis stage is just few hours, but if the substrate is composed of fats and protein, it takes a few days [4]. It is often said that hydrolysis is the limiting step, specifically, when a high lignin feed material is used.
2.4.2. Acidogenesis
Usually, acid production is the second step in all AD process of organic waste. It is possible to divide this stage into acidogenesis and acetogenesis [4]. The fastest reaction is the acidogenesis phase [31, 127]. In this stage monomers or long chain molecules from hydrolytic stage are degraded into volatile fatty acids (VFAs) (e.g. acetic, butyric, propionic and valeric acids), acetate, alcohol and other short-chain fatty acids, H2, alcohols and CO2 by fermentative microorganisms (e.g. Streptococcus, Lactobacillus, Bacillus, Escherichia coli, Salmonella, Clostridium, Ruminococcus, Bacillus, Escherichia, Bacteroïdes, Enterobacter, etc) [15, 138]. These microorganisms are called acidogens or acidogenic bacteria. The precursors for CH4 production in the process are acetic and butyric acids [45, 49]. Acidogenesis is primarily characterized by the buildup of lactate, ethanol, propionate, butyrate and higher VFAs called electron sink or intermediate products [49, 145]. Therefore, bacterium responsible for the hydrolysis and acidogenesis are facultative and obligate anaerobic bacteria [38]. Acidogenesis is followed by acetogenesis, a sub-stage where organic acids and alcohols are converted to acetate together with CO2 and H2 by two co-existing groups of acetogens including, syntrophic acetogenic bacteria producing H2, acetate and CO2 from VFAs and homoacetogens converting CO2 and H2 to acetate [31, 127, 146]. Again, it can be said that Syntrophobacter are propionate-utilizing acetogens while Syntrophomonas are butyrate-utilizing acetogens which are bacteria belonging to the genera Acetobacterium woodii and Clostridium aceticum [15, 49, 146]. Normally, bacteria require time (delay period known as dead time) before processing materials fed to digester tanks before biogas/methane is produced [21]. This period is technically referred to as the lag phase and had been incorporated to almost all microbial growth kinetic models in the literature to estimate the length of time microorganisms spend in reactors before growth and biogas production is observed.
2.4.3. Methanogenesis
Methanogenesis is the most critical as well as rate-limiting stage, all through the AD process that result in long start-ups of up to 3 months step [138, 145]. It constitutes the last stage of AD in which methanogens generate CH4 from the final products of acetogenesis [42]. As CH4 is produced, stability and performance of the process is considered up to par. Methane yield from various feedstock can be measured in m3/kg VS, dm3/kg TS or any simple unit of volume as shown in Table 3.
Table 3: Summary of Biomass and Their Methane Yield [4, 42, 145]
Substrate type | Methane yield (m3/kg VS) | Substrate type | Methane yield (m3/kg VS) |
Iponnoea stem | 0.426 | Grass | 0.190-0.467 |
Poplar wood | 0.33 | Clover grass | 0.290-0.390 |
Hemp | 0.355-0.409 | Switchgrass | 0.140-0.298 |
Sunflower | 0.154-0.400 | Hay | 0.236-0.281 |
Oilseed rape | 0.240-0.340 | Peanut hull | 0.112-0.182 |
Potatoes | 0.275-0.426 | Cauliflower stems | 0.331 |
Potato skin | 0.267 | Beet leaves | 0.231 |
Sugar beet | 0.236-0.381 | Citrus waste | 0.137 |
Fooder beet | 0.420-0.500 | Orange peel | 0.230-0.332 |
Barley | 0.353-0.658 | Maize | 0.259 |
Triticale | 0.337-0.555 | Onion skin | 0.4 |
Alfalfa | 0.340-0.500 | Cucumber waste | 0.309 |
Ryegrass | 0.390-0.410 | Fluted pumpkin peel | 0.161-0.164 |
Nettle | 0.120-0.420 | Cattle slurry | 0.156-0.240 |
Straw | 0.242-0.324 | Water hyacinth | 0.362 |
Leaves | 0.417-0.453 | Pulp and paper mill sludge | 0.429 |
Cattle manure | 0.2-0.25 | Food waste | 0.440-0.480 |
Pig manure | 0.25-0.35 | Kitchen waste | 0.7 |
Poultry manure | 0.3 | Arthrospira platensis | 0.293 |
Carrot waste | 0.417 | Chlamydomonas reinhardtii | 0.387 |
Carrot petioles | 0.309 | Chlorella kessleri | 0.218 |
Banana fruit and stem | 0.529 | Dunaliella salina | 0.42 |
Banana peeling | 0.277-0.411 | Dunaliella salina | 0.323 |
Tomato waste | 0.42 | Spirulina sp. | 0.424 |
Sorghum | 0.42 | Green algae | 0.31 |
Corn stover | 0.36 | Chlorella sp. | 0.264 |
Paddy straw | 0.367 | Monoraphidium sp. | 0.264 |
Millet straw | 0.39 | Chlorella vulgaris | 0.150-0.350 |
Wheat straw | 0.270-0.383 | Isochrysis galbana | 0.338 |
Vegetable waste | 0.19-0.40 | Selenastrum capricornutum | 0.209 |
POME | 0.5-0.55 | Scenedesmus sp. | 0.351 |
Methanogens needs nitrogen to make up for their protein requirements, leading to the production of CH4 by three groups of methanogens, namely acetotrophic, methylotrophic and hydrogenotrophic [129]. Acetotrophic methanogens (include, Methanonococcus mazei, Methanothrix soehngenii and Methanosarcina barkeri) decompose acetate into CH4 and CO2 [45, 69, 127] via the Equation (1).
![]()
Hydrogenotrophic bacteria such as Methanospirillum hungatei and Methanoculles receptaculi consume H2 to yield CH4 [34] via Equation 2 and 3.
![]()
Methylotrophic methanogens changes methyl or trimethylamine specie of a given substrate into CH4 through Equation 4 and 5 pathway [129]:
![]()
Majority of components of biogas are generated during the methanogenic phase, which is seriously affected by temperature, pH, substrate type and feeding rate [4]. Other factors, namely, substrate complexity, process complexity, low productivity, poor stability and inefficient biodegradability impede CH4 production from AD [67, 69]. AD is not the only area bacteria has economic advantage. Oil-eating bacteria can help degrade oil spilled on the environment, a process known as bioremediation [147].
3. Equations to Estimate Biogas Yield
The methane production rate (MPR) and gas production rate (GPR) are two main defining factors of AD performance. To measure biogas yield, inlet tap of a graduated burette containing paraffin oil and the outlet pipe of a biodigester connected to the top of the burette are open. The generated gas will then displace some volume of the paraffin oil in the graduated burette – thus volume of gas produced can then be taken as the volume of biogas yield. By implication, volume of paraffin oil displaced is proportional to the volume of gas yield as described by [131]. In large biogas plants, this volume is recorded daily in m3/day (SI units). In 2016, IRENA computed gas production across a wide range of RTs and temperatures and came up with Equation (6) to evaluate biogas yield per day.
![]()
Where, 𝐺 = biogas production (m3/day), 𝑉𝑑 = digester volume (m3), S = initial conc. of volatile solids in the slurry (kg/m3) and Y = yield factor (based on temperature and feedstock RT). If the yield value is measured in terms of volatile solids, it can be corrected to m3/day units by multiplying it by mass flowrate of the volatile solids in the feed, in accordance with [21] and as given in Equation (7).
![]()
Where ![]() = estimated biogas production rate (m3 biogas/day),
= estimated biogas production rate (m3 biogas/day), ![]() = mass flow rate of volatile solids contained in feed material (kg volatile solid/day), YBG = biogas yield (m3 biogas/kg volatile solid) and V = recorded volume of the biogas (ml). Normalized volume of biogas (mLgVSsubstrate-1day-1) measured from water displacement method, similar to paraffin oil displacement method by [131] can be estimated base on Equation 8 [66],
= mass flow rate of volatile solids contained in feed material (kg volatile solid/day), YBG = biogas yield (m3 biogas/kg volatile solid) and V = recorded volume of the biogas (ml). Normalized volume of biogas (mLgVSsubstrate-1day-1) measured from water displacement method, similar to paraffin oil displacement method by [131] can be estimated base on Equation 8 [66],
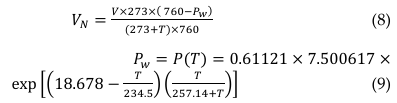
where, 𝑉𝑁 = volume of the dry biogas at standard temperature and pressure (mLN), 𝑃(𝑇) = vapor pressure (mmHg), T = temperature at the ambient space (0C), and Pw = water vapour pressure can be estimated according to the modified Buck equation or Equation (9). Digital biogas flowmeters is more easier to use compared to the displacement method; or the Hohenheim Biogas Yield Test method reported by [148].
3.1. Biogas Yield from Livestock and Crop Residue
Equations to estimate biogas yield for specific feedstock like manures, livestock waste and crop residue has also been presented. For instance, volume of biogas generated annually for manures analyzed in Brazil was estimated using Equation 10 [149],
![]()
where, 𝐼𝑀 = manure generation rate of the animal herd (kg/hr), NA = number of animal herds in all territory, fm = factor of biogas production (m3/t), 1000 = factor for unit adjustment, 𝜀 = collection efficiency in bio-digester {[149] adopted a value of 90%}, and 𝑄𝐵G𝑀 = biogas flow produced by AD of manure (m3/yr). Alternatively, theoretical biogas produced from animal manures can be estimated based on Equation (11) [5, 32, 150],
![]()
where, 𝑇𝑃B = biogas production (m3/yr), M = amount of manure available from the animals (kg/yr), TS = percentage of total solids that can be found in animal manure, AC = coefficient of availability and EBTS = predicted biogas production per kilogram of the total solids (m3/kg TS). Though the content of the livestock waste is not defined, the theoretical biogas production from livestock waste can be predicted using Equation 12 [16],
![]()
where, BP = theoretical biogas potential (TJ y-1), NT = total population of the livestock, VS = volatile solid part of the waste (kg d-1), B0 : CH4 potential of the livestock waste (m3 kg-1), and CV = caloric value of biogas assuming 60% CH4 composition (MJ m-3). Biogas potential from crop residue can be determined using equations 13, 14 and 15 [16],

where, R = total available crop residue (tonnes, t), Cp = amount of crop produced (t y-1), RPR = residue to yield ratio, Y = yield of product (t ha-1 y-1), M = CH4 produced (m3 y-1), TS = total solid (%), VS = volatile solid (%) and MP = methane potential (m3 kg-1 VS). Moles of CH4 gas or biogas produced can be evaluated using the simple ideal gas equation if the pressure, volume and temperature (PVT) conditions are known [151] and is evaluated using Equation (16).
![]()
where, n = number of moles of gas, P = pressure (hPa), V = volume of gas (ml), R = molar gas constant (8.314 J/mol K), and T = temperature (K). Metric volume capacity of CH4 gas production (specific yield) is given in Equation 17 [28].
![]()
Where Vs = CH4 gas metric volume capacity (specific yield) (m3/day), K = kinetic coefficient, B0 = the highest CH4 gas production capacity (m3/kg), S0 = the concentration of volatile solid in the input material (kg/m3), HRT = Hydraulic Retention Time (day), and : maximum specific growth rate of organisms per day.
3.2. Biogas Energy Potential
It is also possible to estimate the amount of electricity that can be generated from biogas, knowing the CH4 content of the gas and the amount of biogas produced yearly. The biogas-generated energy potential can be calculated according to Equation 18 [12],
![]()
where, Ebiogas = quantity of electricity or heat energy produced (kWh year-1), C = lower calorific value of methane (MJ/m3), CH4 = methane content (%), BP = amount of biogas produced per year or the biogas potential (m3 year-1) and 𝜂 = overall efficiency of the conversion of biogas (%). Most potent GHG is CH4 with a greenhouse effect that is 25 times more powerful than CO2 [68]. In the Literature, the main source of greenhouse gas emission of CH4 from AD technology happens as a result of leakage of the reactor and it is up to 5%. The GHG methane emission due to leakage ( ) is presented in Equation 19 [32],
![]()
where, 0.717 kg/m3 = density of CH4 and CH4(AD) = actual volume of CH4 produced from the AD plant. Methane leakage can also be determined using Equation 20 [16],
![]()
where, CH4produced = annual biogas production per digester (m3 y-1) and CE = CH4 collection efficiency. Quantity of CO2 emissions from biogas are predicted according to Equation 21 [16],
![]()
where, CFB = CO2 emission from biogas consumption (tonne of CO2), QBi = quantity of biogas consumed (m3), Ci = calorific value of biogas per unit volume released (TJ m-3), and EFC = carbon emission factor for biogas (tonne TJ-1). A spreadsheet calculator to estimate biogas production and the operational revenue and costs for UK-based farm-fed anaerobic digesters has been developed by [21]. The calculator is first compared with literature reported data, and then applied to the digester unit on a UK Farm to demonstrate its use in a practical setting [21].
4. Utilization of Biogas
The cell gas or typically biogas is employed domestically, locally or for an entire nation in multitude of areas or applications [144]. The gas can be transformed and valorized into diverse forms of energy including power, heat, liquefied natural gas, compressed natural gas or may perhaps be considered as a fuel [152]. Crude biogas is purified using methods including water washing, pressure swing absorption, selexol adsorption, amines gas treatment [1, 143] and/or a simple laboratory setup illustrated in [153]. Particular gaseous species can also be removed, in the case of moisture removal using analytical grade sodium sulphate (Na2SO4), CO2 removal using 15% NaOH solution or iron oxide removal of H2S [92]. Table 4 gives two major areas biogas finds applications.
Table 4: Applications of biogas in the Energy Sector and Biotechnology [21, 39, 70, 154]
Domain | Uses |
Renewable and sustainable energy |
|
Biotechnology |
|
Renewable natural gas (RNG) or biomethane is a methane-rich biogas or biogas that has been upgraded or refined to get rid of CO2, water vapor and traces of other gases to meet natural gas standards [45, 50, 147, 149, 151, 155]. Such gas will then be a substitute for natural gas in applications and can also be upgraded catalytically to synthesis gas [34, 156]. Apart from heating in Table 4, as refined fuel, it finds application in cooking, lighting and running vehicles [27, 34, 136, 145, 157]. For instance, the service of a biogas-powered train named ‘Biogastaget Amanda’ has been employed since 2005 in Sweden; and by 2007, the number of vehicles fueled with upgraded biogas is around 12,000 worlwide. Around 1800s, Louis Pasteur discovered that biomethane could be deployed for lighting and heating; though doing that is wasteful and constitute a pollution [40, 41]. It is fitting to apply biogas for cooking as it is faster than firewood or charcoal stoves [144].
In the chemical industry, upgraded biogas also replaces biogas for chemical production [15, 139]. To minimize cost, gas generators or biogas-powered electricity plants are ambitiously targeted by rural and urban areas of developing and developed countries, especially European nations as alternative renewable energy [15, 28, 137]. Worldwide, installed capacity of biogas for electricity generation is estimated to reach 22,040 megawatts (MW) by 2025 even though economic feasibility is still a major barrier [5, 139]. The co-byproduct of the AD process is the digestate, a slurry which can be sold as fertilizer, specifically called biofertilizer for additional revenue [27, 135]. All these are advantages associated with the production of biogas. Table 5 presents the merits and demerits linked to biogas use and synthesis.
Table 5: Advantages and Disadvantages Governing Production and Utilization of Biogas [15, 41, 45]
Merits | Demerits |
[1]. Substitute for other fuels [2]. Plant occupy small area [3]. Limits greenhouse gases (GHGs) [4]. Avert deforestation [5]. Cheaper technology [6]. Diverse feedstock [7]. Long service years (up to 20 years) [8]. Can be locally built [9]. Sanitation and waste management [10]. As organic fertilizer [11]. Energy for rural and urban inhabitants [12]. Employment creation | [1]. Involves seeding [2]. Microbes are temperature sensitive [3]. Weather sensitive (limited output below 15 ) [4]. Not attractive industrially (not economically feasible) |
It is obvious that disadvantages associated with the use and production of biogas or biomethane is not one that limit its manufacture as more and more plants are built yearly across continents of the globe, because those challenges can easily be addressed.
5. Factors Affecting Biogas Yield
Biogas yield is simply the resulting biogas output per unit mass of substrate or volatile solid [21]. Factors influencing the production of biogas are feedstock type, pH, VFAs, tank volume, RT, pressure, organic loading rate (OLR), chemical oxygen demand (COD), temperature, trace elements/nutrients, carbon to nitrogen (C/N) ratio, inoculation ratio, moisture content, microbial proliferation, pretreatment, additives, alkalinity and particle size [27, 28, 38, 42, 64, 121, 136, 137, 158]. Normally, how these parameters are carefully chosen is crucial to optimizing AD for biogas production [129].
5.1. pH
The most significant parameter affecting the digestion of organic waste feedstock is pH [68]. The alkalinity is a critical indicator of stability and survival of microorganisms in AD process, as it has a great stimulating effect and should be kept within 2500 to 5000 mg/l [141, 145]. A 0.5 pH fluctuation can significantly affect reaction kinetics and gas yield. Hence, in modern plants, pH controllers are installed to check possible fluctuations.
AD occurs at optimum neutral pH of 7 [11, 68, 69]. The fraction of CO2 in the digester gas, concentration of VFA, alkalinity of the system and bicarbonate concentration are compounds/agents causing pH change in digesters [73, 145]. Low pH can inhibit bacterial growth and gas generation causing system failure or low buffering capacity [42]. High pH is no different. Therefore, pH should be kept within a range of 6.0-8.0 [47]. [31] and [38] all reported a value of pH within the range affirmed by [67]. The 3 bacterial degradation stages discussed earlier, functions at specific pH range. Microorganisms involved in hydrolysis require a pH of around 6.0 [68]. Acidogenic bacteria operates at optimum pH of 5.5-6.5 [73, 127]. Acetogenic bacteria survive at pH range of 6.0-7.0 while slow growing methanogenic bacteria requires an optimum pH of 6.5-8.2 [66, 134].
5.2. C/N Ratio
Carbon (C) and nitrogen (N) are one of the most crucial nutrients/elemental compositions to watch during AD fermentation and are scientifically presented as ratios, termed carbon to nitrogen (C/N) ratio [42, 124]. Nitrogen-containing compounds such as nucleic acid, urea or uric acid and proteins are often converted to NH3 during microbial decomposition [33, 58, 69]. In that case, the level of NH3 in the biodigester plays a critical role in the stability of the process [159]. It can act as a pH neutralizer against VFAs. On the contrary, high amount of NH3 intoxicate the microorganisms and increase pH which then inhibit further growth [34, 58, 68].
It is necessary therefore, to examine the effect of low and high C/N ratio in the fermentation process. High C/N ratio depletes nitrogen desired by the AD process, causes slow degradation and result in reduced biogas yield [43, 73, 141]. FW is known to be rich in nitrogen [151]. Low C/N ratio in FW lead the digester to a ‘sour’ situation, reduce pH in the AD system and accumulate inhibitors such as NH3 [43, 51, 141]. For hydrolysis, optimum C/N ratio is between 16/1 and 45/1 while C/N ratio is in the range of 20–35 or 20-30 for methanogenesis [4, 37, 123, 160]. To adjust the C/N ratio, FW from slaughterhouses, MSW and food processing industries that are partly rich in protein and fat can be mixed and fed to the reactor [129]. Natural clinoptilolite have also been used as means to adjust the C/N ratio in AD system [47]. The C/N ratio of some raw materials for AD has been detailed in Table 6.
Table 6: C/N ratio Levels of Various Feed Materials [4, 54, 59, 60, 123, 126, 131]
Substrates/materials | C/N ratio | Substrates/materials | C/N ratio |
Cow dung | 16-25 | Rice straw | 51-67 |
Poultry manure | 5-15 | Wheat straw | 50-150 |
Pig manure | 6-14 | Straw (rice, wheat) | 70 |
Sheep dung | 30-33 | Whole grain | 20-24 |
Elephant dung | 43 | Whole plant ensilage | 35-70 |
Horse manure | 20-40 | Sugar cane bagasse | 140-150 |
Rabbit manure | 17.9 | Corn stalks/straw | 50-56 |
Deer manure | 25.72-30.06 | Oat straw | 48-50 |
Kitchen waste | 25-29 | Sugar beet/sugar foliage | 35-40 |
Fruits and vegetable waste | 7-35 | Fallen leaves | 50-53 |
Food waste | 3-17 | Seaweed | 70-79 |
Peanut shoots/hulls | 20-31 | Algae | 75-100 |
Waste cereals | 16-40 | Sawdust | 200-500 |
Grass/grass trimmings | 12-16 | Potatoes | 35-60 |
Grass ensilage (meadow grass, clover) | 14-22 | Waste from sawmills | 511 |
Alfalfa | 12-17 | Paper | 173 |
Slaughterhouse waste | 22-37 | Human excreta | 8 |
Goat manure | 10-20 | Water hyacinth | 25 |
Mixed FW | 15-32 | Municipal solid waste | 40 |
Sludge | 6 | Mulberry leaves | 14.85 |
Saw dust | >200 | Mushroom residue | 21.96-23.11 |
Silkworm | 11.28 |
5.3. Retention Time
In carrying out design and optimization of AD systems, retention time (RT) is one of the parameters to consider [129]. It is divided into a hydraulic retention time (HRT) taking care of the liquid phase or solid retention time (SRT) denoting the time spent by the microbial culture in the digester [68]. By definition, HRT or hydraulic loading rate (HLR) is the mean time spent by the feedstock in the biofermenter, whereas SRT is the mean time the solid bacteria or microorganism spend in the digester [40, 44, 57, 58, 73]. If the feedstock and microbial mixed cultures are available in the same phase, then HRT = SRT [129]. Mathematically, RT can be evaluated based on the active volume of the digester tank and the volume feed flow rate [21, 27]. Both RTs are presented in Equation 22 and 23,
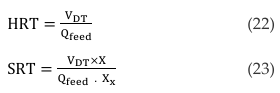
where, VDT = active volume in the digester tank (m3), Qfeed = influent flow rate (m3/day), X = cell concentration in the reactor (mg/l), and XX = concentration in flow out of the reactor (mg/l). Equation 22 is based on [4, 34, 140, 150] while Equation 23 is according to [64]. RT should not be too short or too long. Short RTs constitute a risk of flushing out unfermented feedstock, cell intoxication, accumulation of VFAs, low CH4 yield and even process failure [48]. Long RTs causes slow gas yield, death of microorganisms due to depleted nutrients and insufficient usage of the feedstock [34, 40, 123]. Longer RTs has been reported by [46] to be about 250 days in Austria and 150 days in Germany by law. RTs are sometimes selected based on the ambient temperature condition of the host environment or bacterial survival temperature. The RTs are 10-50 days for mesophilic, 60–120 days in psychrophilic and 14-16 days in thermophilic conditions [68, 77, 161]. Mean RT for animal waste is 20–40 days while for organic waste, it is 60–90 days [42]. Based on the weather condition of tropical and cold countries, RTs fluctuates [68]. Therefore, factors that determines the required RT are process temperature, type of technology and waste composition [161].
5.4. Temperature
Temperature influence the survival of microbes in AD system and affects the rate of biological reactions [120, 128, 141]. It has a direct effect on microbial performance as each bacterial group has a specific temperature range they are active in [21, 162]. Again, the choice of temperature is gravely influenced by the climatic considerations [42]. Bacteria can survive in four different temperature conditions, namely; psychrophilic (10-25 ), mesophilic (20-45 ), thermophilic (45-70 ) and hyperthermophilic ( 70 ) temperature regimes [4, 31, 37, 49, 57, 58, 67, 127, 150, 163].
Higher temperature, especially those between mesophilic and thermophilic regimes is a perfect environment for biological decomposition [68, 141]. Mesophilic enzymes functions at an optimum temperature of 37 [159]. Sometimes too high a reactor temperature leads to process failure and could denature sensitive enzymes. Very low digester temperature slows down AD and may not enhance an optimal catalytic efficiency of the enzymes [37, 67]. For ideal fermentation, the temperature should be kept above 30 [42]. The weather condition is capable of fluctuating the digester temperature, as such, in 2022, Ibrahima Toure developed a digital console to monitor temperature and humidity in bioreactors [3]. Sawdust to serve as insulators to digester tank bodies to maintain a room temperature for digestion had been studied using cotton yarn waste by [114].
Table 7 compares the merits and demerits of the two most common temperature regimes.
Table 7: Mesophilic Versus Thermophilic Process [49, 58, 69, 73, 127, 141]
| Advantages | Drawbacks |
Mesophilic process |
|
|
Thermophilic process |
|
|
Generally, thermophilic range is advantageous over mesophilic range due to its faster degradation rate, high OLR and greater efficiency. The two are however tolerable to 3 temperature fluctuation. But however, requires a continuous reacting system if OLR is the most important factor to the operator. Equation 24 [34, 69] is a correlation between the reaction rate and the biological process with temperature while the Arrhenius equation or Equation 25 [34, 164] gives the temperature dependency on CH4 emission.

Where KT = reaction rate constant at temperature, T, K20 = reaction rate constant at 20 , ![]() = temperature activity constant, T = temperature ( or K), A = pre-exponential (frequency) factor, Ea = activation energy (J mol-1), K = CH4 emission rate (g CH4 / kg VS/d), and R = universal gas constant (8.314 J/mol/K).
= temperature activity constant, T = temperature ( or K), A = pre-exponential (frequency) factor, Ea = activation energy (J mol-1), K = CH4 emission rate (g CH4 / kg VS/d), and R = universal gas constant (8.314 J/mol/K).
5.5. Organic Loading Rate
OLR is defined as the amount of biomass fed into the anaerobic digester per day per unit volume of the system during continuous feed [73, 141]. It is commonly expressed in terms of volatile solids, VS/m3 day, COD, kg/m3 day and total solid, TS/L day [150]. It is possible to calculate OLR using Equation 26 [31, 50], Equation 27 [162] or Equation 28 [159].
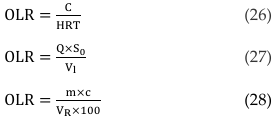
where, C = feed concentration (g. VS/L), HRT = hydraulic retention time, Vl = volume of liquid in the reactor (l), Q = flow rate (l/h), S0 = influent substrate concentration (g COD/L), m = amount of substrate in a unit time (kg d-1), c = concentration of organic dry matter (% oDM), and VR = volume of reactor (m3). [38] and [67] have given a satisfactory detail on OLR. A very high OLR can cause process inhibition by rising the VFAs content, there causing irreversible acidification and failure of the process [69, 161, 162].
5.6. Pressure, COD and Inoculation Ratio
Literature report on the effect of pressure in AD systems is scanty, because majority of biogas storage systems, tank vessels and their covers are limited to holding low pressures (near atmospheric pressure) [129]. Except during compression to be stored in LPG cylinders, after purification and/or H2O removal, that a pressure of up to 4 bar is maintained. However, during co-digestion of cow dung and Tofu liquid waste, [165] noticed that by controlling the internal gas pressure of the bioreactor, CH4 output is found to be higher than the uncontrolled scheme. In another research carried out by [166], when a piston is used to maintain a reduced constant internal gas pressure, amount of CH4 was observed to increase, thereby reducing the cost of chemical addition to maintain a stable pH. The measure of the amount of oxygen needed to oxidize all organic materials present to H2O and CO2 by titrimetric and photometric methods is known as COD [167]. COD content of several feedstock can be determined, in order to optimize the procedure, produce accurate result and improve efficiency of the process [167]. COD:N:Phosphorus (P) ratio had also been reported in the range of 300–600:5:1, which are needed to maintain the digester operation [168]. Inoculum supply to digesters undergoing AD, will help improve the overall gas yield, increase the CH4 volume in biogas and reduce the retention period [169]. The inoculation ratio in AD is described as the substrate-to-microbe (S/M), feedstock-to-inoculum (F/I), or substrate-to-inoculum (S/I) ratio [170]. It can be calculated on the basis of volatile solids, total solids or loading rate. A greater inoculation ratio can lessen the start-up time and enhance the CH4 yield [120].
5.7. Micro and Macro-nutrients
Sufficient amount of both macro- and micronutrients are vital for the continuous performance of the biogas process [150]. Heavy metals or macronutrients have very little effect on VFA composition but pose a major hazard to microbial activity and population [135]. For survival of microbes and stable multistage AD process, low concentrations of trace elements or micronutrients such as tungsten, cobalt, zinc, iron, chromium, nickel, molybdenum, magnesium and selenium are needed [58, 73]. In energy crops, nutrient concentration are inadequate causing problems such as acidification, low CH4 yield and process instability in those crops [150].
5.8. Agitation/mixing
In order to create a homogeneous physical, chemical and biological environment in the anaerobic biodigester, mixing is necessary. Improper mixing destroys microbial cells, disturb the symbiosis of acetogens and methanogens, forms anaerobic granules, and make the process too costly [58, 73]. High speed mixing depletes biogas yields whereas low velocity mixing allows reactor to absorb the disruption due to shock loading [120]. It is beneficial when it is done properly to keep the temperature evenly distributed throughout, maintain a good mass transfer within the system, minimize hydraulic dead zone and foaming in the digester, dilute toxic and inhibitory agents in the reactor bringing them to a uniform composition, balance pH levels and prevent deposition of solids and scum in the AD process, and supply microorganisms with nutrients [4, 28, 58, 141, 145].
Several mixing techniques depending on digester design and substrate type had been described in the literature. Large-scale biogas plants uses pneumatic, mechanical and hydraulic mixing technologies [159]. Mechanical mixers uses shaft in which either impellers or propellers are attached [28]. They are however difficult and costly when installing them for FW. Main advantage of pneumatic mixing technology in which gas sparging is an example is that it decreases the complexity, requires no moving parts and low cost of maintenance [64]. It has the disadvantage of inability to mix around top and bottom of the digester. Installation of mixing devices, such as piston, nozzles and scrapers is another alternative used in some plants; as in Schmidt-Eggersgluss German-type of biogas plant, where nozzle is incorporated to flush slurry so as to achieve a desired mixing effect [169]. Optimal stirring inside the reactor depends on several factors such as size of the digester, mixing technology, operating temperature, the feedstock used and dry matter value of the substrate [159].
5.9. Inhibitors
Examples of inhibitors of detrimental effect to AD process are spices, detergents, mineral ions, oxygen and sulphide. Sulphides are output of sulphate-producing bacteria (SRB). These sulphides, will inhibit SRB or methanogens, accelerate oxidation of organics, decrease CH4 formation, alter the pH value and reduce the efficiency of the methanogenic stage [159]. One example is H2S which is considered poisonous in large quantity but acceptable in low amounts [64]. Heavy metals (iron, nickel, molybdenum, cadmium, lead, mercury, chromium, copper, cobalt and zinc) and light metals (potassium, magnesium, sodium, calcium and aluminum) are among nutrients crucial for survival of microbes in right concentrations [58, 150]. Smaller concentrations have stimulatory effect on microbes while higher concentration inhibit bacterial growth by disrupting the structure and function of the enzymes [58, 147]. Soluble salts of light and heavy metals are toxic substances [42].
Detergents, oxygen and spices could also inhibit AD systems. Detergents in small quantity is safe in anaerobic digesters but lead to death of microbes when in large quantity [120]. Anaerobic mediums require no oxygen; since oxygen are toxic to microorganism surviving in oxygen-free environment [150]. Spices are antimicrobial materials due to the presence of biochemical components such as thymol in thyme and oregano and eugenol in clove, carvacrol in oregano, allicin in garlic, vanillin in vanilla, cinnamic aldehyde in cinnamon and allyl isothiocyanate in mustard [73]. Other toxics are alkylbenzenes, halogenated benzenes, nitro benzenes, phenol, alkylphenols, nitrophenols, halogenated phenols, alcohols, halogenated alcohols, alkanes, aldehydes, ethers, ketones, halogenated aliphatics acrylates, carboxylic acids, amines, nitriles, amides, pyridine and its derivatives, as well as long-chain fatty acids [159].
5.10. VFA Concentration
VFAs are transitional output of CH4 production pathway. The principal acids produced from AD are acetic acid, propionic acid and butyric acid [37, 38, 42, 168]. Accumulation of VFAs lowers the pH level and could occur when the speed of fermentation throughput is much slower compared to their formation [69, 159, 160]. Elevated levels of VFA levels are obviously linked to unconverted organic matter coming along with bad smells [11, 33, 171].
5.11. Moisture Content
Moisture content or H2O content are relevant, in that they aid metabolism and activities of microorganisms. Based on moisture content, there are three types of feedstock used for AD of organic waste, which are wet, semi-dry and dry AD [43, 58]. Dry AD doesn’t require substrate pretreatment into watery pulp and is simpler compared to wet AD [155]. When the moisture content exceeds 20%, dry digestion technology is preferable, especially for FW [150, 155]. A semi-dry AD process is employed for municipal waste recycling plants while wet fermentation technique is engaged in WWTPs. Also, based on the above moisture content type, the feedstock may be in form of high solids, thick slurry and thin slurry [50, 151].
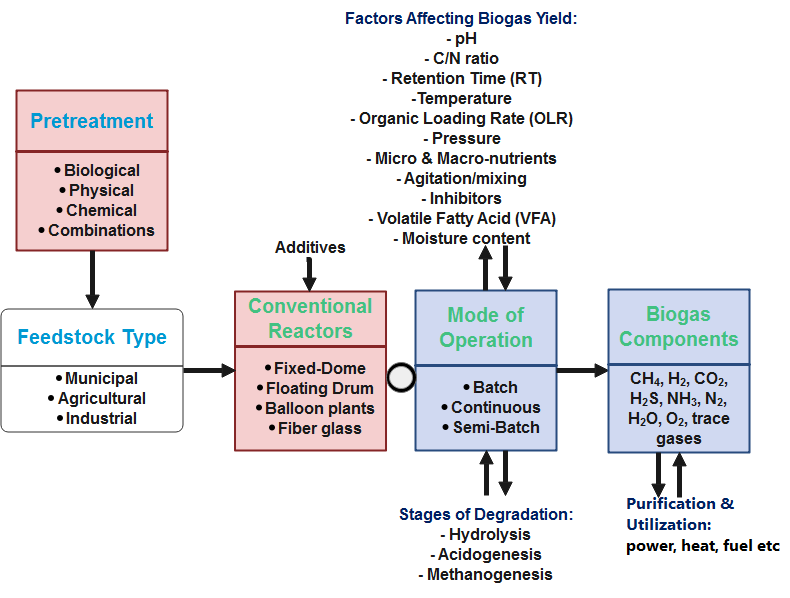
Figure 4 also lists four types of digesters, among which is the floating gas holder type. Versions of the floating drum bioreactor is the Khadi Village Industries Commission (KVIC) model, Deenbandhu model (developed by Action for Food Production (AFPRO) in 1984) and the Pragati model (a combination of Deenbandhu and KVIC designs), all regarded as the Indian digesters [20, 54]. Utilization of the Deenbandhu model in India is around 90% over other models and a 2m3/day capacity of this plant costs $105.36, whereas KVIC will cost $184.38. Different balloon digester development technologies is prominent in application in countries like Belize, Kenya, Peru, Uganda, Bolivia and South Africa which are modifications to the first developed ones in the 1960s in Taiwan [168]. Biogas produced in large scale establishments is mostly stored in double membrane gas storage balloons, with a pressure of 25 bar and total volume of either 1040 m3, 3000 m3 or 4040 m3 [19].
6. Conclusion
From the essential ingredients for a successful transition from biowaste to biogas presented here, it is obvious that lots of expertise is required before venturing into the process and while the process is ongoing. Hence, biogas production is a versatile aspect that requires adequate feasibility studies before setting up a plant and routine laboratory test to maintain a desired environment in the system. Perhaps, that explains the reason why countries that cannot survive the demanding nature and dynamics involve in the process, are yet to find the technology profitable and possibly the reason most of them became non-operational after few years of commissioning. The failure cannot be blamed only on the absence of expertise but also the lack of political will to address the energy needs in those areas, probably attributed to the low economic fortune of those countries. Researchers are however providing enormous inputs in almost all universities in the world to make government see the effectiveness of the process in addressing their energy needs.
The use of aquatic biomass apart from water hyacinth and pharmaceutical wastes are not that prominent compared to other biomass. Further studies are needed to eliminate pharmaceutical waste and invasive aquatic biomass from land and water bodies respectively, in order to solve pollution problems they pose. Modelling and simulation in this area has gained the needed recognition from experts but can still be tabled for further concerns from researchers in relevant fields where limitations are highlighted. Already, several kinetic models of biogas production that can be used to forecast the potentials of a particular substrate for biogas production has been developed. The world is in the 10th decade since the inception of academic research in biogas production, but as old as biogas technology is, only few softwares had been developed so far to facilitate sensitivity analysis, optimization and better simplify the understanding of the process.
Conflict of Interest
The authors declare no conflict of interest.
- O. S. Joshua et al., “Fundamental principles of biogas product,” International Journal of Scientific Engineering and Research (IJSER), vol. 2, no. 8, pp. 47–50, 2014.
- S. Harirchi et al., “Microbiological insights into anaerobic digestion for biogas, hydrogen or volatile fatty acids (VFAs): A review,” Bioengineered, vol. 13, no. 3, pp. 6521–6557, 2022, doi:10.1080/21655979.2022.2035986.
- I. Toure et al., “Design and realization of digital console for monitoring temperature and humidity in a biodigester,” International Advance Journal of Engineering Research (IAJER), vol. 5, no. 2, pp. 1–6, 2022.
- M. R. Atelge et al., “Biogas production from organic waste: Recent progress and perspectives,” Waste and Biomass Valorization, vol. 11, pp. 1–22, 2018, doi:10.1007/s12649-018-00546-0.
- M. M. Ali et al., “Mapping of biogas production potential from livestock manures and slaughterhouse waste: A case study for African countries,” Journal of Cleaner Production, vol. 256, no. 120499, pp. 1–18, 2020, doi:https://doi.org/10.1016/j.jclepro.2020.120499.
- T. M. Thompson, B. R. Young, S. Baroutian, “Advances in the pretreatment of brown macroalgae for biogas production,” Fuel Processing Technology, vol. 195, no. 106151, pp. 1–12, 2019, doi:10.1016/j.fuproc.2019.106151.
- S. Alexopoulos, Biogas systems: Basics, biogas multifunction, principle of fermentation and hybrid application with a solar tower for the treatment of waste animal manure, vol. 5, no. 4, (Kavala Institute of Technology, 2012).
- O. Norouzi, A. Dutta, “The current status and future potential of biogas production from Canada’s organic fraction municipal solid waste,” Energies, vol. 15, no. 475, pp. 1–17, 2022, doi:https://doi.org/10.3390/en15020475.
- M. Talevi et al., “Speaking from experience: Preferences for cooking with biogas in rural India,” Energy Economics, vol. 107, no. 105796, pp. 1–10, 2022, doi:https://doi.org/10.1016/j.eneco.2021.105796.
- V. P. Aravani et al., “Agricultural and livestock sector’s residues in Greece & China: Comparative qualitative and quantitative characterisation for assessing their potential for biogas production,” Renewable and Sustainable Energy Reviews, vol. 154, no. 111821, pp. 1–10, 2022, doi:https://doi.org/10.1016/j.rser.2021.111821.
- O. Kucher et al., “Energy potential of biogas production in Ukraine,” Energies, vol. 15, no. 5, pp. 1–22, 2022, doi:https://doi.org/10.3390/en15051710.
- T. S. Nam et al., Lab-scale biogas production from co-digestion of super-intensive shrimp sludge and potential biomass feedstocks, vol. 6, no. 1, (2022).
- I. Syofii, D. P. Sari, “Production of biogas based on human fesses as an alternative energy for remote areas application,” Indonesian Journal of Engineering and Science, vol. 3, no. 1, pp. 1–6, 2022, doi:https://doi.org/10.51630/ijes.v3.i1.29.
- P. Mukumba et al., “A possible design and justification for a biogas plant at Nyazura Adventist High School, Rusape, Zimbabwe,” Journal of Energy in Southern Africa, vol. 24, no. 4, pp. 12–21, 2013.
- L. Ioannou-ttofa et al., “Life cycle assessment of household biogas production in Egypt: Influence of digester volume, biogas leakages, and digestate valorization as biofertilizer,” Journal of Cleaner Production, vol. 286, no. 125468, pp. 1–14, 2021, doi:10.1016/j.jclepro.2020.125468.
- E. W. Gabisa, S. H. Gheewala, “Potential, environmental, and socio-economic assessment of biogas production in Ethiopia: The case of Amhara regional state,” Biomass and Bioenergy, vol. 122, pp. 446–456, 2019, doi:10.1016/j.biombioe.2019.02.003.
- H. Gebretsadik, S. Mulaw, G. Gebregziabher, “Qualitative and quantitative feasibility of biogas production from kitchen waste,” American Journal of Energy Engineering, vol. 6, no. 1, pp. 1–5, 2018, doi:10.11648/j.ajee.20180601.11.
- A. M. Abubakar, “Biodigester and feedstock type: Characteristic, selection, and global biogas production,” Journal of Engineering Research and Sciences (JENRS), vol. 1, no. 3, pp. 170–187, 2022, doi:https://doi.org/TBA.
- S. Gençoğlu, M. S. Bilgili, “Management of liquid digestate in biogas plants,” 9th Global Conference on Environmental Studies (CENVISU-2021) Antalya, Turkey, no. 14, pp. 51–62, 2021.
- Mohamed Samer, “Biogas plant constructions,” in Biogas, (Cairo, Egypt: , 2012), 344–368, doi:10.5772/31887.
- A. Wu et al., “A spreadsheet calculator for estimating biogas production and economic measures for UK-based farm-fed anaerobic digesters,” Bioresource Technology, vol. 220, pp. 479–489, 2016, doi:10.1016/j.biortech.2016.08.103.
- K. Rajendran et al., “A novel process simulation model (PSM) for anaerobic digestion using Aspen Plus,” Bioresource Technology, 2014.
- J. A. Arzate, “Modeling and simulation of biogas production based on anaerobic digestion of energy crops and manure,” (Technischen Universitat Berlin, Berlin, 2019).
- H. T. T. Nong et al., “Development of sustainable approaches for converting the agro-weeds Ludwigia hyssopifolia to biogas production,” Biomass Conversion and Biorefinery, pp. 1–9, 2020, doi:https://doi.org/10.1007/s13399-020-01083-4.
- O. Khayal, “Main types and applications of biogas plants,” Nile Valley University, pp. 1–11, 2019, doi:10.13140/RG.2.2.32559.69287.
- R. Jyothilakshmi, S. V Prakash, “Design, fabrication and experimentation of a small scale anaerobic biodigester for domestic biodegradable solid waste with energy recovery and sizing calculations,” Procedia Environmental Sciences, vol. 35, pp. 749–755, 2016, doi:10.1016/j.proenv.2016.07.085.
- IRENA, “Measuring small-scale biogas capacity and production.” Abu Dhabi, United Arab Emirates, 2016.
- M. A. Fahriansyah, Sriharti, “Design of conventional mixer for biogas digester,” IOP Conference Series: Earth and Environmental Science, pp. 1–8, 2019, doi:10.1088/1755-1315/277/1/012017.
- Z. Lenkiewicz, M. Webster, “How to convert organic waste into biogas: A step-by-step guide.” wasteaid.org.uk/toolkit . (accessed: 14-Aug-2021).
- M. M. Jaffar, A. M. Rehman, “Wheat straw optimization via its efficient pretreatment for improved biogas production,” Civil Engineering Journal, vol. 6, no. 6, pp. 1056–1063, 2020, doi:http://dx.doi.org/10.28991/cej-2020-03091528.
- D. Elalami et al., “Pretreatment and co-digestion of wastewater sludge for biogas production: Recent research advances and trends,” Renewable and Sustainable Energy Reviews, vol. 114, no. 109287, pp. 1–23, 2019, doi:10.1016/j.rser.2019.109287.
- T. Chowdhury et al., “Latest advancements on livestock waste management and biogas production: Bangladesh’s perspective,” Journal of Cleaner Production, vol. 272, no. 122818, pp. 1–20, 2020, doi:https://doi.org/10.1016/j.jclepro.2020.122818.
- Y. Lahlou, Design of a biogas pilot unit for Al Akhawayn University (School of Science and Engineering, 2017).
- B. Bharathiraja et al., “Biogas production – A review on composition, fuel properties, feed stock and principles of anaerobic digestion,” Renewable and Sustainable Energy Reviews, vol. 90, pp. 570–582, 2018, doi:10.1016/j.rser.2018.03.093.
- EIA, “Biomass explained.” www.eia.gov-energyexplained-biomass-landfill-gas-and-biogas-php . (accessed: 14-Aug-2021).
- P. M. Njeru, P. Njogu, “Conversion of water hyacinth derived biogas to biomethane for electricity generation in Kenya: A waste to energy (WtE) approach,” Proceedings of 2014 International Conference on Sustainable Research and Innovation, vol. 5, pp. 79–81, 2014.
- 37 Fuchs et al., “Tackling ammonia inhibition for efficient biogas production from chicken manure: Status and technical trends in Europe and China,” Renewable and Sustainable Energy Reviews, vol. 97, pp. 186–199, 2018, doi:10.1016/j.rser.2018.08.038.
- A. Neshat et al., “Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production,” Renewable and Sustainable Energy Reviews, vol. 79, pp. 308–322, 2017, doi:10.1016/j.rser.2017.05.137.
- M. M. Esteves et al., “Life cycle assessment of manure biogas production: A review,” Journal of Cleaner Production, vol. 219, pp. 411–423, 2019, doi:10.1016/j.jclepro.2019.02.091.
- 40 V Yentekakis, G. Goula, “Biogas management: Advanced utilization for production of renewable energy and added-value chemicals,” Frontiers in Environmental Science, vol. 5, pp. 1–16, 2017, doi:10.3389/fenvs.2017.00007.
- L. Granado et al., “Technology overview of biogas production in anaerobic digestion plants: A European evaluation of research and development,” Renewable and Sustainable Energy Reviews, vol. 80, pp. 44–53, 2017, doi:10.1016/j.rser.2017.05.079.
- 42 A. Raja, S. Wazir, “Biogas production: The fundamental processes,” Universal Journal of Engineering Science, vol. 5, no. 2, pp. 29–37, 2017, doi:10.13189/ujes.2017.050202.
- 43 HomeBioGas, “What is Biogas? A Beginner’s Guide.” www.homebiogas.com/what-is-biogas-a-beginners-guide- . (accessed: 13-Aug-2021).
- 44 Parsaee et al., “A review of biogas production from sugarcane vinasse,” Biomass and Bioenergy, vol. 122, pp. 117–125, 2019, doi:10.1016/j.biombioe.2019.01.034.
- 45 M. A. Abuabdou et al., “A review of anaerobic membrane bioreactors (AnMBR) for the treatment of highly contaminated land fill leachate and biogas production: Effectiveness, limitations and future perspectives,” Journal of Cleaner Production, vol. 255, no. 120215, pp. 1–12, 2020, doi:10.1016/j.jclepro.2020.120215.
- Stürmer et al., “Agricultural biogas production: A regional comparison of technical parameters,” Renewable Energy, vol. 164, pp. 171–182, 2021, doi:10.1016/j.renene.2020.09.074.
- A. Abd, M. R. Othman, “Biogas upgrading to fuel grade methane using pressure swing adsorption: Parametric sensitivity analysis on an industrial scale,” Fuel, vol. 308, no. 121986, pp. 1–10, 2022, doi:https://doi.org/10.1016/j.fuel.2021.121986.
- Aryal et al., “Microbial electrochemical approaches of carbon dioxide utilization for biogas upgrading,” Chemosphere, vol. 291, no. 132843, pp. 1–10, 2022, doi:https://doi.org/10.1016/j.chemosphere.2021.132843.
- Yuan et al., “A review on biogas upgrading in anaerobic digestion systems treating organic solids and wastewaters via biogas recirculation,” Bioresource Technology, vol. 344, no. 126412, pp. 1–10, 2022, doi:https://doi.org/10.1016/j.biortech.2021.126412.
- Jung et al., “Upgrading biogas into syngas through dry reforming,” Renewable and Sustainable Energy Reviews, vol. 143, no. 110949, pp. 1–10, 2021, doi:https://doi.org/10.1016/j.rser.2021.110949.
- A. Westenbroek, J. I. Martin, “Anaerobic digesters and biogas safety.” https://farm-energy.extension.org/anaerobic-digesters-and-biogas-safety/ . (accessed: 11-Mar-2021).
- K. Sheryazov et al., “Study of the parameters of biogas plants,” IOP Conference Series: Earth and Environmental Science, DAICRA 2021, vol. 949, no. 012108, pp. 1–11, 2022, doi:10.1088/1755-1315/949/1/012108.
- F. I. Al Imam et al., “Development of biogas processing from cow dung, poultry waste, and water hyacinth,” International Journal of Natural and Applied Science, vol. 2, no. 1, pp. 13–17, 2013.
- Oxfam, “Design, construction and maintenance of a biogas generator.” Oxfam Technical Briefs, : 1–10, 2015.
- 55 B. Møller et al., “Agricultural biogas production — Climate and environmental impacts,” Sustainability, vol. 14, no. 1849, pp. 1–24, 2022, doi:https://doi.org/ 10.3390/su14031849.
- Mazurkiewicz, “Energy and economic balance between manure stored and used as a substrate for biogas production,” Energies, vol. 15, no. 413, pp. 1–17, 2022, doi:https://doi.org/10.3390/en15020413.
- Ł. Małgorzata, J. Frankowski, “The biogas production potential from silkworm waste,” Waste Management, vol. 79, pp. 564–570, 2018, doi:10.1016/j.wasman.2018.08.019.
- M. Abubakar, M. U. Yunus, “Reporting biogas data from various feedstock,” International Journal of Formal Sciences: Current and Future Research Trends (IJFSCFRT), vol. 11, no. 1, pp. 23–36, 2021, doi:10.5281/zenodo.6366775.
- 59 Chaump et al., “Leaching and anaerobic digestion of poultry litter for biogas production and nutrient transformation,” Elsevier, vol. 84, pp. 413–422, 2018, doi:10.1016/j.wasman.2018.11.024.
- 60 J. Patinvoh et al., “Innovative pretreatment strategies for biogas production,” Bioresource Technology, vol. 224, pp. 13–24, 2016, doi:10.1016/j.biortech.2016.11.083.
- 61 L. de C. e Silva et al., “Lab-scale and economic analysis of biogas production from swine manure,” Renewable Energy, vol. 186, pp. 350–365, 2022, doi:10.1016/j.renene.2021.12.114.
- 62 Grasselli, T. Gal, J. Szendrei, “Possibilities to establish biogas plants in the Northern Great Plain Region, based on cattle and pig manure,” University of Debrecen, Centre for Agricultural Sciences and Engineering, pp. 85–87, 2008.
- 63 Aromolaran, M. Sartaj, R. M. Z. Alqaralleh, “Biogas production from sewage scum through anaerobic co‑digestion: The effect of organic fraction of municipal solid waste and landfill leachate blend addition,” Biomass Conversion and Biorefinery, pp. 1–17, 2022, doi:10.1007/s13399-021-02152-y.
- 64 K. Srivastava, “Advancement in biogas production from the solid waste by optimizing the anaerobic digestion,” Waste Disposal & Sustainable Energy, vol. 2, no. 2, pp. 85–103, 2020, doi:10.1007/s42768-020-00036-x.
- 65 Wongarmat et al., “Anaerobic co-digestion of biogas effluent and sugarcane filter cake for methane production,” Biomass Conversion and Biorefinery, vol. 12, pp. 901–912, 2022, doi:https://doi.org/10.1007/s13399-021-01305-3.
- 66 Achinas, G. J. W. Euverink, “Elevated biogas production from the anaerobic co-digestion of farmhouse waste: Insight into the process performance and kinetics,” Waste Management & Research, vol. 37, no. 12, pp. 1240–1249, 2019, doi:10.1177/0734242X19873383.
- 67 Zhang, K. Loh, J. Zhang, “Enhanced biogas production from anaerobic digestion of solid organic wastes: Current status and prospects,” Bioresource Technology Reports, vol. 5, pp. 280–296, 2019, doi:10.1016/j.biteb.2018.07.005.
- 68 K. Pramanik et al., “The anaerobic digestion process of biogas production from food waste: Prospects and constraints,” Bioresource Technology Reports, vol. 8, pp. 1–38, 2019, doi:10.1016/j.biteb.2019.100310.
- 69 Mirmohamadsadeghi et al., “Biogas production from food wastes: A review on recent developments and future perspectives,” Bioresource Technology Reports, vol. 7, no. 100202, pp. 1–37, 2019, doi:10.1016/j.biteb.2019.100202.
- 70 Brémond et al., “Biological pretreatments of biomass for improving biogas production: An overview from lab scale to full-scale,” Renewable and Sustainable Energy Reviews, vol. 90, pp. 583–604, 2018, doi:10.1016/j.rser.2018.03.103.
- 71 Sivaprakash et al., “Analysis of bio-gas from kitchen waste,” International Conference on Advances in Materials, Computing and Communication Technologies 2385, vol. 090001, no. January, pp. 1–7, 2022, doi:https://doi.org/10.1063/5.0070710 Published.
- 72 Kaya, M. Bİlgİn, “Biogas production from potato peel,” Acta Biologica Turcica, vol. 35, no. 1, pp. 49–56, 2022.
- 73 Sahu et al., “Evaluation of biogas production potential of kitchen waste in the presence of spices,” Waste Management, vol. 70, pp. 236–246, 2017, doi:10.1016/j.wasman.2017.08.045.
- 74 M. El-Dalatony et al., “Pig‑ and vegetable-cooked waste oils as feedstock for biodiesel, biogas, and biopolymer production,” Biomass Conversion and Biorefinery, pp. 1–11, 2022, doi:10.1007/s13399-021-02281-4.
- 75 Jupesta, “Using biogas from palm oil residues to enhance energy access in Indonesia,” International Association for Energy Economics, pp. 33–35, 2015.
- 76 C. Ortega-bravo et al., “Biogas production from concentrated municipal sewage by forward osmosis, micro and ultrafiltration,” Sustainability, vol. 14, no. 2629, pp. 1–11, 2022, doi:https://doi.org/10.3390/su14052629.
- 77 C. Caruso et al., “Recent updates on the use of agro-food waste for biogas production,” Applied Sciences, vol. 9, no. 6, pp. 1–29, 2019, doi:10.3390/app9061217.
- 78 F. Al Ajlouni, M. Khattab, “Biogas and compost generated from organic waste at Al-Akaider landfill in Jordan,” Journal of Advanced Sciences and Engineering Technologies (JASET), vol. 5, no. 1, pp. 8–22, 2022, doi:https://doi.org/10.32441/jaset.05.01.02.
- 79 Ben Oumarou et al., “Statistical modelling of the energy content of municipal solid wastes in Northern Nigeria,” Arid Zone Journal of Engineering, Technology and Environment (AZOJETE), vol. 12, pp. 103–109, 2016.
- 80 B. Oumarou, A. M. Kundiri, M. Dauda, “Material recovery from wastes: An employment and poverty alleviation tool,” Arid Zone Journal of Engineering, Technology and Environment (AZOJETE), vol. 13, no. 1, pp. 66–76, 2017.
- 81 M. Kundiri, B. A. Umdagas, M. B. Oumarou, “Characterization of leachate contaminants from waste dumpsites in Maiduguri, Borno State,” Arid Zone Journal of Engineering, Technology and Environment (AZOJETE), vol. 13, no. 1, pp. 140–148, 2017.
- 82 Priadi et al., “Biogas production in the anaerobic digestion of paper sludge,” 2013 5th International Conference on Chemical, Biological and Environmental Engineering (ICBEE 2013), vol. 9, pp. 65–69, 2014, doi:10.1016/j.apcbee.2014.01.012.
- 83 Williams, “The production of bioethanol and biogas from paper sludge,” (Stellenbosch University, 2017).
- 84 Rodriguez et al., “Mechanical pretreatment of waste paper for biogas production,” Waste Management, vol. 68, pp. 157–164, 2017, doi:10.1016/j.wasman.2017.06.040.
- 85 Patra, “Aquatic weed resources in India and South-East Asia,” International Journal of Current Research (IJCR), vol. 5, no. 5, pp. 1343–1345, 2013.
- 86 N. Ismail et al., “Invasive aquatic plant sciences of Chenderoh Reservoir, Malaysia,” IOP Conference Series: Earth and Environmental Science, pp. 1–8, 2019.
- 87 Winardi Dwi Nugraha, Syafrudin, and Lathifah Laksmi Pradita, “Biogas production from water hyacinth,” in Biogas-Recent advances and integrated approaches, eds Abd El-Fatah Abomohra et al. (InTech Open, 2020), 15, doi:10.5772/intechopen.91396.
- 88 Kawaroe et al., “Utilization of aquatic weed Salvinia molesta as a raw material for biogas production,” Jurnal Pengolahan Hasil Perikanan Indonesia (JPHPI), vol. 22, no. 2, pp. 209–217, 2019, doi:https://doi.org/10.17844/jphpi.v22i2.27891.
- 89 O. Ezama, “Impact of water hyacinth infection in Nigeria inland waters: Utilization and Management,” (The Maritime Commons: Digital Repository of the World Maritime University, Malmo, Sweden, 2019).
- 90 D. F. Langa, M. P. Hill, S. G. Compton, “Agents sans frontiers: cross-border aquatic weed biological control in the rivers of Southern Mozambique,” African Journal of Aquatic Science, vol. 45, no. 3, pp. 329–335, 2020, doi:https://doi.org/10.2989/16085914.2020.1749551.
- 91 Arne Jernelov, “Water hyacinths in Africa and Asia,” in The lon-term fate of invasive species, (2017), 117–136, doi:10.1007/978-3-319-55396-2_9.
- 92 Njogu et al., “Biogas production using water hyacinth (Eicchornia crassipes) for electricity generation in Kenya,” Energy and Power Engineering, vol. 7, pp. 209–216, 2015, doi:http://dx.doi.org/10.4236/epe.2015.75021.
- 93 D. Nugraha et al., “Biogas production from water hyacinth (Eichhornia crassipes): The effect of F/M ratio,” 8th International Conference on Future Environment and Energy (ICFEE 2018), pp. 1–6, 2020, doi:10.1088/1755-1315/150/1/012019.
- 94 B. Barua, A. S. Kalamdhad, “Water hyacinth to biogas: A review,” Pollution Research Paper, vol. 35, no. 3, pp. 491–501, 2016.
- 95 A. Enaboifo, O. C. Izinyon, “Potential of biogas production from floating aquatic weeds,” Periodical: Advanced Materials Research, vol. 824, pp. 467–472, 2013, doi:https://doi.org/10.4028/www.scientific.net/AMR.824.467.
- 96 A. Jimin, E. I. Magani, H. I. Usman, “Rainy season identification and species characteristics of aquatic macrophytes in the floodplains of River Benue at Markudi,” Journal of Sustainable Development in Africa, vol. 16, no. 6, pp. 145–165, 2014.
- 97 A. Eyo, “Review of possibilities of water hyacinth (Eichhornia crassipes) utilization for biogas production by rural communities in Kainji Lake Basin.” http://hdl.handle.net/1834/18845 . (accessed: 07-Apr-2022).
- 98 Hussner, “Alien aquatic plant species in European countries,” Weed Research, vol. 52, no. 4, pp. 297–306, 2021, doi:https://doi.org/10.1111/j.1365-3180.2012.00926.x.
- 99 Hussner et al., “Management and control methods of invasive alien freshwater aquatic plants: A review,” Aquatic Botany, vol. 136, pp. 112–137, 2017, doi:http://dx.doi.org/10.1016/j/aquabot/2016/08.002.
- 100 Bochmann et al., “Anaerobic digestion of pretreated industrial residues and their energetic process integration,” Bioprocess Engineering, vol. 19, pp. 1–11, 2020, doi:https://doi.org/10.3389/fbioe.2020.00487.
- 101 Rasel et al., “Industrial waste management by sustainable way,” Europa Journal of Engineering and Technology Research (EJ-ENG), vol. 4, no. 4, pp. 111–114, 2019, doi:http://dx.doi/10.24018/ejers.2019.4.4.1225.
- 102 Semple, R. Hewett, D. Balzano, “Analysis: Europe’s water/wastewater in numbers.” waterworld.com/international/utilities/article/16201111/analysis-europes-waterwastewater-in-numbers . (accessed: 07-Apr-2022).
- 103 E. Macedo et al., “Global distribution of wastewater treatment plants and their released effluents into rivers and streams,” Earth System Science Data, pp. 1–33, 2022, doi:https://doi.org/10.5194/essd-2021-214.
- 104 Budiyono et al., “Production of biogas from organic fruit waste in anaerobic digester using ruminant as the inoculum,” MATEC Web of Conferences, vol. 156, no. 03053, pp. 1–5, 2018, doi:https://doi.org/10.1051/matecconf/201815603053.
- 105 David O Olukanni, Gbemisola I Megbope, and Oluwatosin J Ogundare, “Assessment of biogas generation potential of mixed fruits solid waste,” in Biomethane through resource circularity, 1st ed. , (CRC Press, 2021), 12.
- 106 M. Alikhan, S. Khoramnejadian, S. M. Khezri, “Vegetable and fruit market wastes as an appropriate source for biogas production via anaerobic digestion process,” Zeitschrift fur Physikalische Chemie, vol. 235, no. 11, pp. 1447–1453, 2021, doi:https://doi.org/10.1515/zpch-2020-1723.
- 107 Aryanto et al., “Utilization of fruit waste as biogas plant feed and its superiority compared to landfill,” International Journal of Technology (IJTech), vol. 8, no. 8, pp. 1385–1392, 2017, doi:https://doi.org/10.14716/ijtech.v8i8.739.
- 108 A. Hammid, N. Aini, R. Selaman, “Anaerobic digestion of fruit wastes for biogas production,” International Journal of Advance Research and Innovative Ideas in Education (IJARIIE), vol. 5, no. 4, pp. 34–38, 2019.
- 109 O. Chinwendu et al., “The potential of biogas production from fruit wastes (watermelon, mango and pawpaw),” World Journal of Advanced Research and Reviews (WJARR), vol. 1, no. 3, pp. 52–65, 2019, doi:https://doi.org/10.30574/wjarr.2019.1.3.0026.
- 110 J. K. Kell, “Anaerobic co-digestion of fruit juice industry wastes with lignocellulosic biomass,” (Stellenbosch University, 2019).
- Sharda Dhadse and Shanta Satyanarayan, “Role of microbial and organic amendments for the enrichment of methane production in bioreactor,” in Biogas: Basics, integrated approaches, and case studies Working Title, eds Abd El-Fatah Abomohra and El-Sayed Salama (InTech Open, 2022), doi:10.5772/intechopen.102471.
- 112 Lu et al., “Biogas generation in anaerobic wastewater treatment under tetracycline antibiotic pressure,” Scientific Report, vol. 6, no. 28336, pp. 1–9, 2016, doi:10.1038/srep28336.
- P. Juanga-Labayen, I. V Labayen, Q. Yuan, “A review on textile recycling practices and challenges,” Textiles, vol. 2, pp. 174–188, 2022, doi:https://doi.org/10.3390/textiles2010010.
- Twizerimana et al., “Anaerobic digestion of cotton yarn wastes for biogas production: Feasibility of using sawdust to control digester temperature at room temperature,” Scientific Research, vol. 8, no. 7, pp. 1–15, 2021, doi:10.4236/oalib.1107654.
- Opwis, J. S. Gutmann, “Generation of biogas from textile waste waters,” Chemical Engineering Transactions (CEt), vol. 27, pp. 103–108, 2012, doi:10.3303/CET1227018.
- 116 Kumar, S. Samuchiwal, A. Malik, “Anaerobic digestion of textile industries wastes for biogas production,” Biomass Conversion and Biorefinery, vol. 10, pp. 715–724, 2020, doi:https://doi.org/10.1007/s13399-020-00601-8.
- Cruz-Salomon et al., “Biogas production from a native beverage vinasse using a modified UASB bioreactor,” Portal Komunikacji Naukowej, vol. 198, pp. 170–174, 2017, doi:10.1016/j.fuel.2016.11.046.
- 118 Wickham et al., “Anaerobic digestion of soft drink beverage waste and sewage sludge,” Bioresource Technology, vol. 262, pp. 141–147, 2018.
- Mirmohamadsadeghi et al., “Pretreatment of lignocelluloses for enhanced biogas production: A review on influencing mechanisms and the importance of microbial diversity,” Renewable and Sustainable Energy Reviews, vol. 135, no. 110173, pp. 1–19, 2021, doi:10.1016/j.rser.2020.110173.
- 120 Kainthola, A. S. Kalamdhad, V. V Goud, “A review on enhanced biogas production from anaerobic digestion of lignocellulosic biomass by different enhancement techniques,” Process Biochemistry, vol. 84, pp. 81–90, 2019, doi:10.1016/j.procbio.2019.05.023.
- Wacławek et al., “Disintegration of wastewater activated sludge (WAS) for improved biogas production,” Energies, vol. 12, no. 21, pp. 1–15, 2019, doi:10.3390/en12010021.
- Kong et al., “Large pilot-scale submerged anaerobic membrane bioreactor for the treatment of municipal wastewater and biogas production at 25◦C,” Bioresource Technology, vol. 319, pp. 1–12, 2021, doi:10.1016/j.biortech.2020.124123.
- M. M. N. Dehkordi et al., “Investigation of biogas production potential from mechanical separated municipal solid waste as an approach for developing countries (case study: Isfahan-Iran),” Renewable and Sustainable Energy Reviews, vol. 119, no. 109586, pp. 1–12, 2020, doi:10.1016/j.rser.2019.109586.
- Banerjee, N. Prasad, S. Selvaraju, “Reactor design for biogas production: A short review,” Journal of Energy and Power Technology (JEPT), vol. 4, no. 1, pp. 1–14, 2022, doi:10.21926/jept.2201004.
- 125 O. Olatunji et al., “Modelling the effects of particle size pretreatment method on biogas yield of groundnut shells,” Waste Management & Research (WMR), pp. 1–13, 2022, doi:10.1177/0734242X211073852.
- 126 Sarto, R. Hildayati, I. Syaichurrozi, “Effect of chemical pretreatment using sulfuric acid on biogas production from water hyacinth and kinetics,” Renewable Energy, vol. 132, pp. 335–350, 2019, doi:10.1016/j.renene.2018.07.121.
- 127 Abraham et al., “Pretreatment strategies for enhanced biogas production from lignocellulosic biomass,” Bioresource Technology, vol. 301, no. 122725, pp. 1–13, 2020, doi:10.1016/j.biortech.2019.122725.
- 128 Hagos et al., “Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives,” Renewable and Sustainable Energy Reviews, vol. 76, pp. 1485–1496, 2017, doi:http://dx.doi.org/10.1016/j.rser.2016.11.184.
- 129 Sarker et al., “A review of the role of critical parameters in the design and operation of biogas production plants,” Applied Sciences, vol. 9, no. 9, pp. 1–38, 2019, doi:10.3390/app9091915.
- 130 Yu et al., “A review of crop straw pretreatment methods for biogas production by anaerobic digestion in China,” Renewable and Sustainable Energy Reviews, vol. 107, pp. 51–58, 2019, doi:10.1016/j.rser.2019.02.020.
- 131 M. Uche et al., “Design and construction of fixed dome digester for biogas production using cow dung and water hyacinth,” African Journal of Environmental Science and Technology, vol. 14, no. 1, pp. 15–25, 2020, doi:10.5897/AJEST2019.2739.
- 132 H. Chowdhury, “Review on the pre-treatment methods of waste for anaerobic digestion,” The Seu Journal of Electrical and Electronic Engineering (SEUJEEE), vol. 2, no. 1, pp. 46–51, 2022.
- 133 Liu et al., “Effects of liquid digestate pretreatment on biogas production for anaerobic digestion of wheat straw,” Bioresource Technology, vol. 280, pp. 345–351, 2019, doi:10.1016/j.biortech.2019.01.147.
- 134 Almomani, “Prediction of biogas production from chemically treated co-digested agricultural waste using artificial neural network,” Fuel, vol. 280, no. 118573, pp. 1–13, 2020, doi:10.1016/j.fuel.2020.118573.
- 135 Mancini et al., “Increased biogas production from wheat straw by chemical pretreatments,” Renewable Energy, vol. 119, pp. 608–614, 2018, doi:10.1016/j.renene.2017.12.045.
- 136 Vinzelj et al., “Employing anaerobic fungi in biogas production: Challenges & opportunities,” Bioresource Technology, vol. 300, no. 122687, pp. 1–10, 2020, doi:10.1016/j.biortech.2019.122687.
- 137 K. Show et al., “Insect gut bacteria: A promising tool for enhanced biogas production,” Reviews in Environmental Science and Bio/Technology, pp. 1–25, 2022, doi:https://doi.org/10.1007/s11157-021-09607-8.
- 138 O. Wagner, P. Illmer, “Biological pretreatment strategies for second-generation lignocellulosic resources to enhance biogas production,” Energies, vol. 11, no. 1797, pp. 1–14, 2018, doi:10.3390/en11071797.
- 139 A. Rajput, C. Visvanathan, “Effect of thermal pretreatment on chemical composition, physical structure and biogas production kinetics of wheat straw,” Journal of Environmental Management, vol. 221, pp. 45–52, 2018, doi:10.1016/j.jenvman.2018.05.011.
- Tabatabaei et al., “A comprehensive review on recent biological innovations to improve biogas production, Part 1: Upstream strategies,” Renewable Energy, vol. 146, pp. 1204–1220, 2019, doi:https://doi.org/10.1016/j.renene.2019.07.037.
- 141 Raymond, U. Okezie, “The significance of biogas plants in Nigeria’s energy strategy,” Journal of Physical Sciences and Innovation, vol. 3, pp. 11–17, 2011.
- Guo et al., “Biodegradation of guar gum and its enhancing effect on biogas production from coal,” Fuel, pp. 1–9, 2021, doi:10.1016/j.fuel.2021.122606.
- 143 Pigosso et al., Fundamentals of anaerobic digestion, biogas purification, use and treatment of digestate (Concordia, SC: Brazilian Agricultural Research Corporation-Embrapa Swine & Poultry, 2022).
- K. McCabe, T. Schmidt, Integrated Biogas Systems: Local Applications of Anaerobic Digestion Towards Integrated Sustainable Solutions (Queensland, Australia: IEA Bioenergy, 2018).
- 145 Singh, Z. Szamosi, Z. Siménfalvi, “Impact of mixing intensity and duration on biogas production in an anaerobic digester: A review,” Critical Reviews in Biotechnology, vol. 40, no. 4, pp. 508–521, 2020, doi:10.1080/07388551.2020.1731413.
- P. C. Bong et al., “The characterisation and treatment of food waste for improvement of biogas production during anaerobic digestion– A review,” Journal of Cleaner Production, vol. 172, pp. 1545–1558, 2017, doi:10.1016/j.jclepro.2017.10.199.
- M. Abubakar, M. Alhassan, “History, adverse effect and clean up strategies of oil spillage,” International Journal of Applied Sciences: Current and Future Research Trends (IJASCFRT), vol. 11, no. 1, pp. 31–51, 2021, doi:10.5281/zenodo.5557307.
- V Miroshnichenko, N. V Nikulina, “Designing a biogas plant for an educational and scientific livestock complex,” 8th Scientific and Practical Conference ”Biotechnology: Science and Practice”, pp. 151–163, 2022, doi:10.18502/kls.v7i1.10117.
- F. S. dos Santos et al., “Assessment of potential biogas production from multiple organic wastes in Brazil: Impact on energy generation, use, and emissions abatement,” Resources, Conservation & Recycling, vol. 131, pp. 54–63, 2018, doi:10.1016/j.resconrec.2017.12.012.
- Sawyerr et al., “An overview of biogas production: Fundamentals, applications and future research,” International Journal of Energy Economics and Policy, vol. 9, no. 2, pp. 105–116, 2019, doi:10.32479/ijeep.7375.
- Maroušek et al., “Advances in nutrient management make it possible to accelerate biogas production and thus improve the economy of food waste processing,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, pp. 1–10, 2020, doi:10.1080/15567036.2020.1776796.
- Tabatabaei et al., “A comprehensive review on recent biological innovations to improve biogas production, Part 2: Mainstream and downstream strategies,” Renewable Energy, vol. 146, pp. 1392–1407, 2019, doi:https://doi.org/10.1016/j.renene.2019.07.047.
- 153 Oladejo et al., “Pretreatment and optimization strategy for biogas production from agro-based wastes,” The International Journal of Engineering and Science (IJES), vol. 11, no. 3, pp. 14–20, 2022, doi:10.9790/1813-1103011420.
- Venturin et al., “Effect of pretreatments on corn stalk chemical properties for biogas production purposes,” Bioresource Technology, vol. 266, pp. 1–36, 2018, doi:10.1016/j.biortech.2018.06.069.
- Khalil et al., “Waste to energy technology: The potential of sustainable biogas production from animal waste in Indonesia,” Renewable and Sustainable Energy Reviews, vol. 105, pp. 323–331, 2019, doi:10.1016/j.rser.2019.02.011.
- Schiaroli et al., “Catalytic upgrading of clean biogas to synthesis gas,” Catalysts, vol. 12, no. 109, pp. 1–28, 2022, doi:https://doi.org/10.3390/catal12020109.
- Z. Abdul, A. M. Abubakar, “Potential swing to natural gas-powered electricity generation,” International Journal of Natural Sciences: Current and Future Research Trends (IJNSCFRT), vol. 10, no. 1, pp. 27–36, 2021.
- 158 Rafiee et al., “Biogas as an energy vector,” Biomass and Bioenergy, vol. 144, no. 105935, pp. 1–10, 2021, doi:https://doi.org/10.1016/j.biombioe.2020.105935.
- Nsair et al., “Operational parameters of biogas plants: A review and evaluation study,” Energies, vol. 13, no. 15, pp. 1–27, 2020, doi:10.3390/en13153761.
- 160 Maroušek et al., “Advances in the agrochemical utilization of fermentation residues reduce the cost of purpose-grown phytomass for biogas production,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, pp. 1–11, 2020, doi:10.1080/15567036.2020.1738597.
- N. Usman, M. A. Suleiman, M. I. Binni, Anaerobic digestion of agricultural wastes: A potential remedy for energy shortfalls in Nigeria, vol. 4, no. 1, (Scholarena, 2021).
- Bakraoui et al., “Biogas production from recycled paper mill wastewater by UASB digester: Optimal and mesophilic conditions,” Biotechnology Reports, vol. 25, pp. 1–8, 2020, doi:10.1016/j.btre.2019.e00402.
- O. Cinar et al., “Long-term assessment of temperature management in an industrial scale biogas plant,” Sustainability, vol. 14, no. 612, pp. 1–17, 2022, doi:https://doi.org/10.3390/su14020612.
- H. Bhatt, L. Tao, “Economic perspectives of biogas production via anaerobic digestion,” Bioengineering, vol. 7, no. 74, pp. 1–19, 2020, doi:10.3390/bioengineering7030074.
- R. I. Utami et al., “Analysis of the effect of internal gas pressure of an anaerobic digester on biogas productivity of a mixture of cow dung and tofu liquid waste,” The 9th National Physics Seminar-AIP onference Proceedings 2320, vol. 2320, pp. 1–9, 2021, doi:https://doi.org/10.1063/5.0037446.
- R. Prajapati, R. K. Sharma, I. M. Amatya, “Effect of reduced gas pressure on yield of biogas and other physicochemical parameters,” International Journal of Energy and Environmental Engineering, vol. 12, pp. 31–37, 2021, doi:https://doi.org/10.1007/s40095-020-00351-3.
- M. W. Harnadek, N. G. H. Guilford, E. A. Edwards, “Chemical oxygen demand analysis of anaerobic digester contents,” STEM Fellowship Journal, vol. 1, no. 2, pp. 1–5, 2015, doi:10,17975/sfj-2015-008.
- 168 Y. Choong, K. W. Chou, I. Norli, “Strategies for improving biogas production of palm oil mill effluent (POME) anaerobic digestion: A critical review,” Renewable and Sustainable Energy Reviews, vol. 82, pp. 2993–3006, 2018, doi:10.1016/j.rser.2017.10.036.
- V. Kumar et al., “A review on production of biogas, fundamentals, applications and its recent enhancing techniques,” Elixir International Journal, vol. 57, pp. 14073–14079, 2013.
- Khadka et al., “Effect of the substrate to inoculum ratios on the kinetics of biogas production during the mesophilic anaerobic digestion of food waste,” Energies, vol. 15, no. 834, pp. 1–16, 2022, doi:https://doi.org/10.3390/en15030834.
- Ghiandelli, “Development and implementation of small-scale biogas balloon biodigester in Bali, Indonesia,” (KTH School of Industrial Engineering and Management, 2017).
- Mishra et al., “Multidimensional approaches of biogas production and up-gradation: Opportunities and challenges,” Bioresource Technology, vol. 338, no. 125514, pp. 1–14, 2021, doi:https://doi.org/10.1016/j.biortech.2021.125514.
- Abdulhalim Musa Abubakar, Kiman Silas, Mohammed Modu Aji, “Biodigester and Feedstock Type: Characteristic, Selection, and Global Biogas Production”, Journal of Engineering Research and Sciences, vol. 1, no. 3, pp. 170–187, 2022. doi: 10.55708/js0103018

