A Review on the Effect of Varied Sand Types in Concrete at High Temperature
Journal of Engineering Research and Sciences, Volume 1, Issue 4, Page # 38-47, 2022; DOI: 10.55708/js0104005
Keywords: Aggregates, Behavior, Concrete, High temperature, Sand
(This article belongs to the Section Civil Engineering (CVE))
Export Citations
Cite
Hachemi, S. and Rahmouni, Z. E. (2022). A Review on the Effect of Varied Sand Types in Concrete at High Temperature. Journal of Engineering Research and Sciences, 1(4), 38–47. https://doi.org/10.55708/js0104005
Samya Hachemi and Zine Elabidine Rahmouni. "A Review on the Effect of Varied Sand Types in Concrete at High Temperature." Journal of Engineering Research and Sciences 1, no. 4 (April 2022): 38–47. https://doi.org/10.55708/js0104005
S. Hachemi and Z.E. Rahmouni, "A Review on the Effect of Varied Sand Types in Concrete at High Temperature," Journal of Engineering Research and Sciences, vol. 1, no. 4, pp. 38–47, Apr. 2022, doi: 10.55708/js0104005.
In fact, aggregates in concrete generally occupied a considerable proportion of volume (60%-75%); sand constitutes about 30% to 50% of aggregates volume. It is well known that the nature of aggregates plays an important role on quality and properties of concrete. This suggests that the behavior of concrete exposed to high temperature is strongly linked to the nature and mineralogy of aggregates (coarse and fine aggregates). Furthermore, the description of the effect of high temperature on the components of concrete is intended to improve understanding of how concrete responds when it is exposed to elevated temperature. The fire performance of concrete depends on the thermal, physical and mechanical properties of its components. Sand can be classified into two groups according to its mineralogical nature: Siliceous and Calcareous, these two types of sand undergo different reactions when they are exposed to high temperature. Few studies have been published and showed that the nature of sand affects the concrete behavior at high temperature. This paper summaries the states-of-the-art studies on the mechanical and physical behavior of concrete made with different types of sand after being exposed to elevated temperature. It is revealed that the fire-response of concrete made with calcareous sand is different from that of concrete made with siliceous sand.
1. Introduction
Hardened concrete is a heterogeneous multi-phases material consisting of a mixture of aggregates, occupying 60 to 75% of concrete volume and hydrated cement paste which represents 25 to 40% of concrete volume. Each component plays a well-defined role, that of filling attenuator of volume variations (recessed and rising in temperature and source of mechanical strength for aggregates) and that of binder and gives the concrete material its properties of rigidity and resistance for cement paste [1].
For a good concrete mix, fine aggregates need to be clean, hard, strong, and free of absorbed chemicals and other fine materials that could cause the deterioration of concrete. Unfortunately, majority of the natural sand used (rolled sand: sand of river, dune sand, and sand of sea) is selected for the price and the availability [2, 3]. Properties of sand affect the durability and performance of mortar, as fine aggregates is an essential component of concrete.
In [4], the author showed that manufactured sand is a best alternative for natural sand in terms of strength and durability and the concrete mix with 60% replacement has given good durable properties. Tebbal [5] studied the effect of using crushed sand (CS) as partial replacement of dune sand (DS) in various percentages (0, 1/3, 2/3, and 100%) on the physic-mechanical properties of HPC made with binary natural fine aggregates (DS and CS) at aggressive environment. Different types of high performance concrete (HPC) are made of using materials and products manufactured in Algeria: Portland Artificial CPJ CEMII 42.5 cement, superplasticizer (SP), two fractions of gravel (3/8 mm) and (8/15 mm) mm and two fillers (silica fume and granulated slag) with two types of sand CS (0/5 mm) and DS (0/5 mm). The experimental study shows that the parameters of workability of HPC are improved when the CS is partially replaced by the DS (<2/3). However, for high content of DS (>1/3), additional quantities of water is needed to meet the workability properties. The mechanical strengths decrease by adding the DS to CS, but they reach acceptable values with CS in moderate dosages. The HPC performances are significantly better than the control concrete made up with the same aggregates. The specification tests of durability show that the water absorbing coefficients by capillarity increase after adding DS to the CS [5].
In the recent years, there were often fires in tunnels and buildings which cause very serious consequences in terms of human and economic losses. When concrete is subjected to high temperature, the material is the seat of numerous degradation processes. Among them, the aggregate-paste incompatibility may have a direct influence on the material stability [6].
The behavior of concrete structures exposed to high temperature depends on many simultaneously interacting factors ranging from composition of materials to the characteristics of fire and stress conditions.
Aggregates play an important role in concrete because they constitute the skeleton through which the efforts are transmitted. Under the effect of temperature, the aggregates decompose and undergo significant chemical and mineralogical transformations that modify the micro-structural characteristics of the material. Generally, the majority of aggregates used for making concrete are stable up to 500°C [7].
Recent studies have reported and showed that the nature of aggregates affects concrete behavior at high temperature [8–10]. Sand can be classified into two groups: Siliceous and Calcareous. These two natures of fine aggregates undergo different reactions when they are exposed to high temperature. Up to 300°C, commonly used aggregate materials are thermally stable. The quartz of siliceous sand can cause cracking at the paste-aggregates interface at about 575°C, due to the crystal transformation of quartz α to quartz β which is associated with a volume expansion of the order of 1%–5.7% [11, 12]. A similar distress can begin above 700°C in the case of carbonate sand, where calcium carbonate (CaCO3) starts to decompose into free lime (CaO) and carbon dioxide (CO2).
In [13], the author was interested to the effect of high temperature on color and residual compressive strength of concrete. This study identified the relationship between temperature change and a change in color and a decrease in compressive strength in a heat exposed concrete structure. For this study, concrete specimens were manufactured and heated to variable temperatures (100°C, 200°C, 300°C, 400°C, 500°C, 600°C, 700°C, and 800°C). Whereupon, the color change and the residual compressive strength were measured for analysis. The result of the study shows that color change and residual compressive strength in concrete structure temperature have a consistent relationship; therefore, it may be possible to know how much compressive strength of concrete exposed to high temperature reduces by measuring color changes and estimating heating temperature [13].
The purpose of this paper is to identify the relationship between the sand type of concrete and their behavior at high temperatures. Six types of concrete are made of using materials and products manufactured in Algeria: Portland Artificial CPJ CEMII 42.5 cement, superplasticizer (SP), two fractions of gravel 15 mm and 25 mm and two types of sand: Siliceous Sand (SS) and Calcareous Sand (CS).
2. Test Programs
2.1. Materials
The experimental investigation of some mechanical and physical properties is carried out on concrete with two different types of sand: CS and SS. The specific densities of these two types of sand measured in the laboratory according to standard NF P 18-554 [14] are 2600 kg/m3 and 2200 kg/m3, respectively. The maximum size for the two types of sand was 5 mm; the particle size distribution is shown in figure 1. SS and CS physical properties are summarized in table 1.
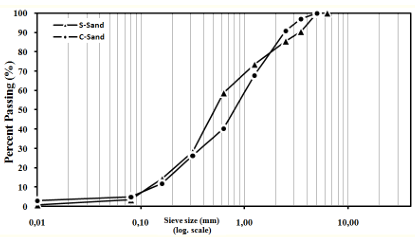
Table 1. Physical properties of SS and CS [15].
Properties | S-Sand | C-Sand |
Apparent density (g/cm3) Absolute density (g/cm3) Sand equivalent (%) Finesse modulus Water content (%) Water absorption (%) | 1.70 2.60 85.0 2.65 0.35 1.03 | 1.64 2.20 54.0 2.63 0.20 3.92 |
All mixtures are made with Portland cement (CPJ CEM II/A 42.5) and coarse calcareous aggregates with a maximum size of 25 mm (specific density is about 2630 kg/m3). The mixture proportions of the different concretes are presented in Table 2. The water used for the different concrete mixtures comes from the laboratory tap.
There were six series, concrete with fine aggregate as SS and concrete with fine aggregate as CS and each series comprised of many cubic specimens. Cubic specimens (100×100×100 mm3) were made and cured under water at room temperature until test time, then, they were removed from water and dried for 2 next days in room temperature (for minimizing the risk of spalling of concrete).
Table 2. Mix proportion of concrete (kg/m3) [15].
Mix | w/c | C | w | SS | CS | Aggregate | SP (%) | ||
25 mm | 15 mm | ||||||||
SS-C1 | 0.60 | 329 | 199 | 715 | – | 646 | 390 | – | |
SS-C2 | 0.42 | 475 | 199 | 715 | – | 646 | 390 | – | |
SS-C3 | 0.27 | 610 | 168 | 715 | – | 646 | 390 | 1.50 | |
CS-C1 | 0.60 | 329 | 199 | – | 715 | 646 | 390 | – | |
CS-C2 | 0.42 | 475 | 199 | – | 715 | 646 | 390 | – | |
CS-C3 | 0.27 | 610 | 168 | – | 715 | 646 | 390 | 1.50 | |
Specimens were placed in an electrical furnace and heated at a constant rate of 3°C/min [16]; they were allowed to cool naturally to room temperature inside the electrical furnace in order to prevent thermal shock. Physical and mechanical tests were performed on unheated and heated samples in order to compare the initial and residual properties.
2.2. Physical properties
The specimens were weighted in different states using an electronic digital balance for determining the mass loss Mloss, water porosity P and density D according to the standard NF EN 12390-7 [17]. The dimensions of width, length and height of concrete specimens were measured, before and after heating to get the variation of specimen’s volume Vv. These properties were determined according to the following Equations:

where:
Minitial is the initial mass (before heating);
Mheated is the heated mass (after heating) weighed in the air;
Msat is the saturated mass measured in the air;
Msat+imm is the saturated mass measured in the water;
V20 is the volume of specimen before heating;
Vt is the volume of specimen after heating.
2.3. Mechanical properties
For compressive strength, uniaxial compression tests were performed on cubic specimens using a hydraulic press according to the standard NF EN 12390-3 [18]. Relative values of compressive strength were expressed as a ratio between strength after heating and strength of unheated material.
The ultrasonic pulse velocity UPV value was determined for quick checking the quality of concrete specimens before and after heating. UPV test were performed in accordance with the standard AFNOR P 18-418 [19]. If the path length is known, then the UPV can be calculated from the path length divided by the transit time.
3. Effects of high temperature on SS and CS (according to the literature)
This part presents an analysis of the physical properties and macroscopic observations of two types of sand after their exposure to heating-cooling cycles. The results obtained provide more knowledge of the changes induced on sand by temperature.
Figure 2 presents the results of a differential thermal analysis (DTA) that allows determining, by endothermic and exothermic peaks, the temperatures at which instability can occurs in aggregates. According to research of Felicetti [7], the majority of aggregates used for the manufacture of concrete are stable up to 500°C.
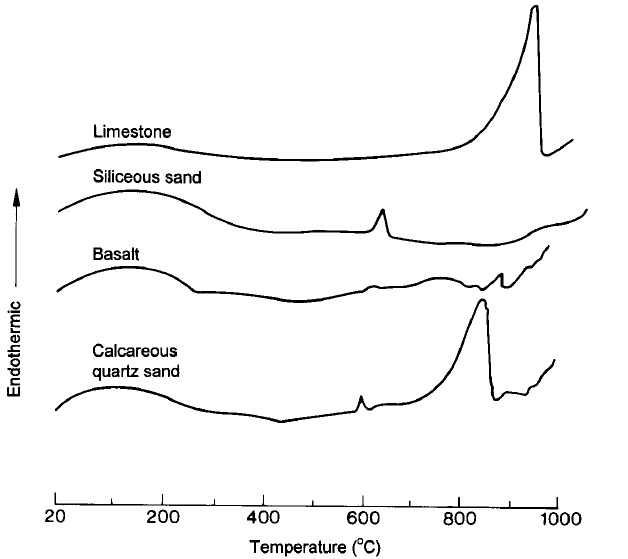
SS contains approximately 20% of bound water. Between 120°C and 600°C, this water is able to partially release by reducing the resistance of this material. In fact, the quartz present in the siliceous aggregates is a building of tetrahedral (a silicon atom surrounded by four oxygen atoms) which undergoes a slight rotation of the bonds to form a crystalline structure of hexagonal symmetry starting from a temperature of 575°C. This variation in the chemical structure of quartz (transformation of α-quartz into β-quartz) is accompanied by a dilatation in the order of 1 to 5.7% [7, 20–22]. Both of these phenomena can cause damage to the concrete structure.
3.1. Macroscopic observations of the effects of temperature on sand
The two types of sand are intact after the heat treatment of the samples to 150°C, no macroscopic degradation was observed. From this temperature, changes on some grain were observed compared to the initial state (20°C). The descriptions of these changes as well as the photographs of the samples before and after the heat treatment are given in figures 3 and 4.
3.1.1. Siliceous Sand SS
The grains of SS remain intact up to 400°C. From this temperature, a variation in the color of SS grains has been observed. Slight and gradual reddening occurs throughout the increase in heating temperature (600°C and 900°C). This red coloration is explained by the dehydration of goethite (iron hydroxide FeO(OH)) which transforms into ferric oxide (Fe2O3) from 300°C [23]. Photographs of SS samples before and after heat treatment are summarized in Figure 3.
3.1.2. Calcareous Sand CS
According to the figure 4, it is clearly noticed that the color of calcareous sand grains remains unchanged until 600°C.
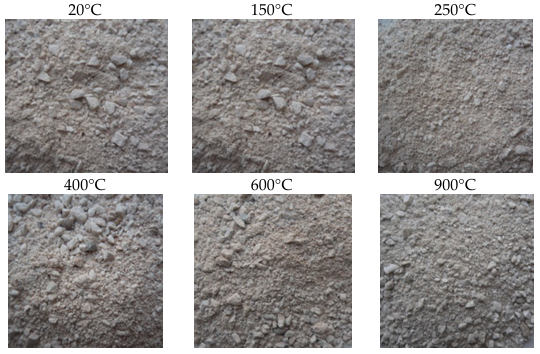
After the heating of 900°C, the majority of the CS grains becoming gray. The formation of the white layer on the surfaces of CS is the consequence of the decarbonation of the limestones, the calcite (CaCO3) transforming itself into lime (CaO) following the departure of CO2 [25–28]. After cooling, the CaO reacts with the moisture of the air and transforms into portlandite Ca(OH)2 [29].
After heating cycles of 600 to 900°C, the increase of the thickness of decarbonated layer with increasing temperature has been observed.
3.2. Mass loss of sand as a function of temperature
The mass losses of CS and SS are grouped in Figure 5. The mass losses of CS and SS were determined by weighing the samples before and after each heating-cooling cycle.
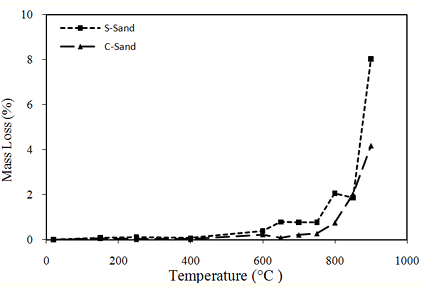
Mass loss values are negligible (less than 0.2%) for temperature cycles of 150°C, 250°C and 400°C. Beyond 600°C, the mass loss of sands increases.
CS samples lose less mass than samples of SS. This difference in mass loss can be attributed to the departure of water trapped in the micro -porosity of SS. The mass loss of CS samples is approximately steady at up to 800°C (less than 1%). After heating to 900°C, the mass loss of CS is twice as low as that of SS.
Calcareous aggregates exhibit stable thermal behavior up to a temperature of 650°C [20]. Beyond this temperature, CaCO3 decomposes (decarbonation) giving carbon dioxide (CO2) and calcium monoxide (CaO). During the cooling process, the free lime (CaO) can react with moisture to give Portlandite Ca(OH)2 with a 40% volume increase [21]. This reaction leads to increased cracking and damage to the concrete structure, which may explain the decrease in residual mechanical resistance for limestone aggregates heated above 700°C. These results confirm the research of Tsymbrovska [30] and Tebbal [31].
4. Effects of high temperature on concretes prepared with different type of sand
Many literatures of effects of high temperature on concretes concluded that when aggregates are subjected to a rise of temperature, they can present thermal instabilities (mineralogical modifications and thermal expansion) which strongly influence the behavior of concrete tempered with these aggregates during a rise of the temperature. It is therefore important to know the behavior of aggregates at high temperatures to facilitate the understanding concrete behavior at high temperature.
4.1. Residual compressive strength
The compressive strength at 28 days is the main characteristic used to distinguish between different types of concrete. It allows understanding the process of degradation of concretes subjected to high temperature. The residual compressive strengths of concrete prepared with siliceous sand (SS-C1, SS-C2 and SS-C3) are compared to the residual compressive strengths of concrete prepared with calcareous sand (CS-C1, CS-C2 and CS-C3).
Figure 6 shows the evolution of the residual and relative compressive strength as a function of the heating temperature.
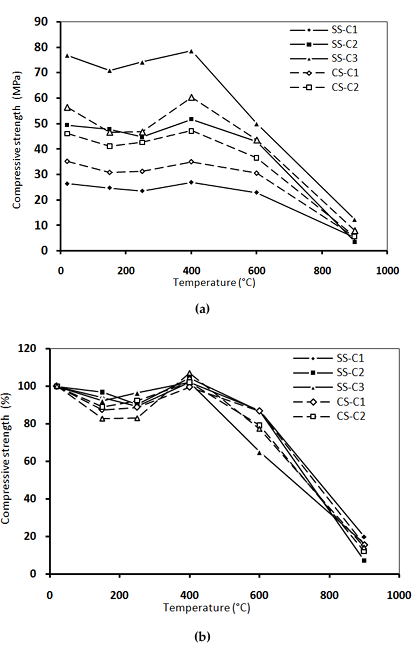
compressive strength (b) with temperature [15].
The evolution of the compressive strength of different concretes with temperature has three distinct phases. From 20 to 250°C, concretes show a slight decrease in compressive strength. This first phase can be explained by the thermal expansion of water. This dilation weakens the bonds between the leaves of the HSCs and discards them. Hager [32] explained this phase by the dehydration of ettringite takes place, followed by the decomposition of gypsum between 150°C and 170°C. Between 200°C and 250°C, slight variations in flux to the continuous dehydration of C-S-H, a so-called “water plug” develops in concrete pores [33].
Around 400°C, the compressive strength increases. This phase is attributed to the departure of water which allows a re-increase of attraction forces and the approximation of CSH leaves. This gain in resistance has been observed by many researchers [15, 24, 25, 31].
Above 400°C, the compressive strength decreases more rapidly. In this range, cement paste contracts, whereas aggregates expand. Therefore, the transition zone and bonding between aggregates and paste are weakened [34].
For concrete contained calcareous sand (CS-C1 with w/c=0,6), an improvement of about 30% in the residual compressive strength compared to SS-C1 is noted in the temperature range of 20°C to 600°C. The nature of the phase changes will depend upon the mineralogical composition of cement, its C/S ratio (mols of lime per mol of silica; CaO/SiO2), the amount of fine particles (quartz) and the temperature and pressure levels that have been reached [35].
The influence of the presence of calcareous sand in concrete brought to high temperature depends on the ratio w/c. This sand improves the residual compressive strength of concretes C1 with ratio of w/c = 0.6 and decreases those of concretes C2 and C3 whose w/c ratio are 0.42 and 0.27, respectively. The improvement in the compression behavior of CS-C1 concrete seems to be related to the absorption coefficient of the CS sand used. This sand has a higher absorption coefficient than SS, which leads to a decrease in the amount of mixing water and consequently an improvement in compressive strength. This result is similar to research of Tebbal [5].
From Figure 5, it can be seen that SS-C3 concrete made from SS has better room temperature resistance than CS-C3. After heating up to 400°C, figure 5 shows a better evolution of residual compressive strength of SS-C3 concrete and a less evolution for concrete CS-C3. This can be explained by the higher density and low absorption of SS that leads to a stronger concrete [25].
At room temperature (20°C), the presence of CS modified slight the compressive strength of CS-C3 concrete. This concrete has a compressive strength lower about 27% than that of SS-C3 concrete with SS. The use of CS for the preparation of high performance concrete does not improve the compressive strength of concrete.
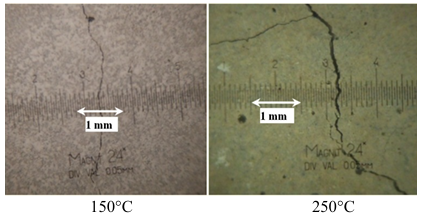
Between 150°C and 250°C, the decrease in residual compressive strength was greater after heating. The decreases of compressive strength at these temperatures were 34% and 37%, respectively. In other hand, the specimens of CS-C3 at the same temperatures show greater damage by cracking compared to SS-C1 concretes.
The photos in Figure 7 show the surface conditions of CS-C3 concrete after heating to 150°C and 250°C. The cracks have been visible to the naked eye and the opening of these cracks is greater than SS-C3 concrete treated at the same temperatures.
These results join the observations made by other researchers like Rahmouni [36] which indicates that concrete containing CS present the higher loss of compressive strength (figure 8).
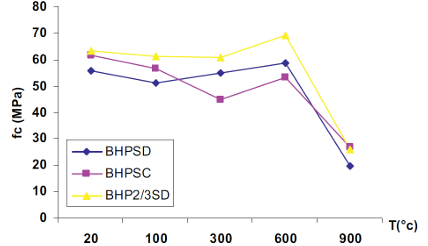
Note : BHPSD: HPC with 100 % of SD. BHPSC: with 100 % of CS . BHP2/3SD: with 2/3 of SD.
Furthermore, Rahmouni [36] pointed out that the loss of compressive strength of high performance concrete contained SS remains moderate up to 600°C and accelerates beyond this temperature. The best compression behavior of concrete with SS can be explained by an improvement of adhesion resistance of interface aggregate/paste. The low w/c ratio indeed leads to a decrease in the porosity of the transition zone.
It was reported also in reference [36] that between 600°C and 900°C, the loss of compressive strength of high performance concrete prepared with silico-calcareous sand is due to spalling and bursting of flint.
4.2. UPV
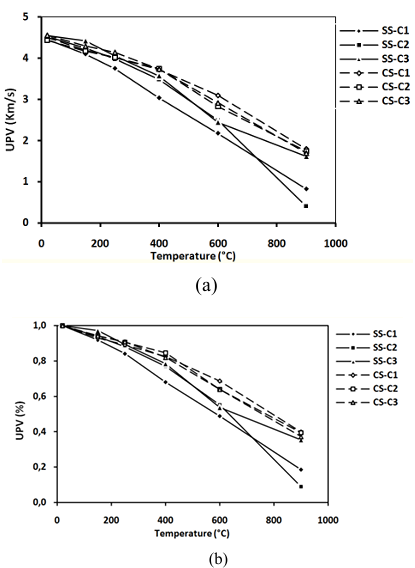
Measurement of UPV is one of the various non-destructive test methods used to obtain the maximum information on the quality of concretes subjected to a rise in temperature.
Figure 9 shows the evolution of UPV of concretes tested after exposed to high temperature. The measurements were repeated three times for each specimen who gave 9 measurements for each point shown in the figure. The UPV of concretes made with SS or CS sand is very affected by the rise in temperature. The decrease of UPV values means an increase of concrete degree damage [37]. However, the UPV values of concretes contained CS are better than those of concretes containing SS.
4.3. Mass loss
Figure 10.a illustrates the evolution of mass loss of concretes contained SS and CS as a function of the heating temperature [15]. The second figure (10.b) shows the effect of high temperature on the evolution of the mass loss of three concretes, these concretes mixes were prepared with different fine aggregates (sand): CS, SS or silico-calcareous sand reported by Rahmouni [36].
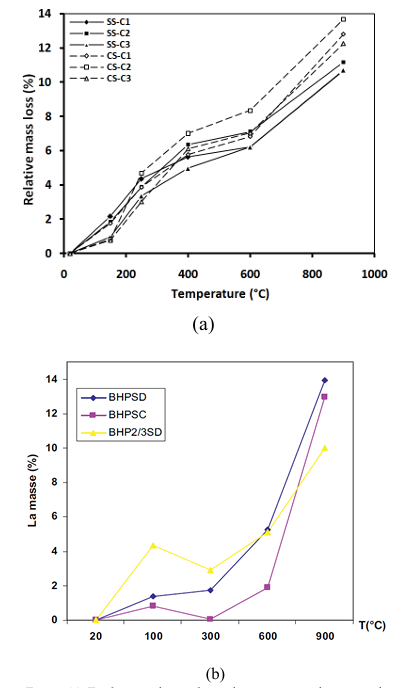
Above 250°C, the evolution of mass loss as a function of temperature is related to the type of sand used [15, 36]. Concretes containing SS have the lowest mass loss compared to concretes containing CS. The greatest mass loss of concretes containing CS can be explained by the departure of CO2 from calcium carbonates which cause an increase in mass loss from 600°C [38].
4.4. Porosity
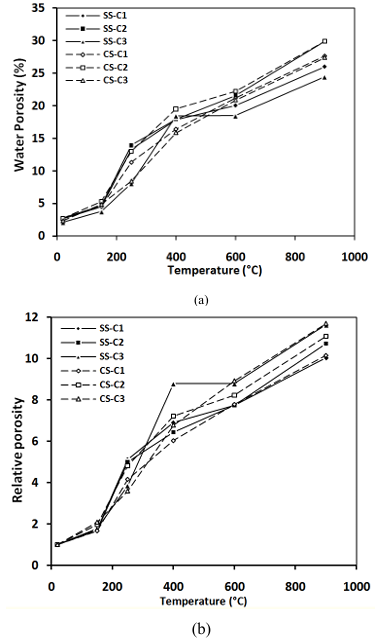
Figure 11 shows the evolution of water porosity of concretes containing CS compared to that of concretes containing SS.
The porosity of concrete is strongly modified with temperature.
Above 400°C, the concrete prepared with CS has a little higher porosity than concrete containing SS. This is due to the decarbonation of calcite (CaCO3) which is transformed into lime (CaO) following the departure of CO2. The rehydration of CaO by the presence of water is accompanied by significant swelling. This increase in volume is responsible for the additional cracking and decohesion of aggregates with the cement past. Similar behaviors had already been observed experimentally by Xing [38].
4.5. Density
Figure 12 shows the evolution of density obtained on all the concretes studied as a function of the heat treatment.
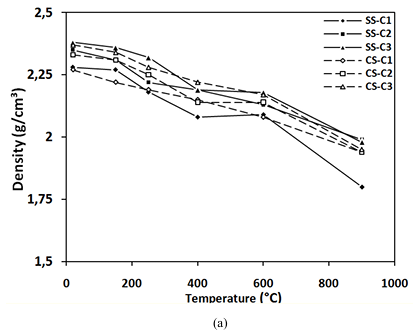
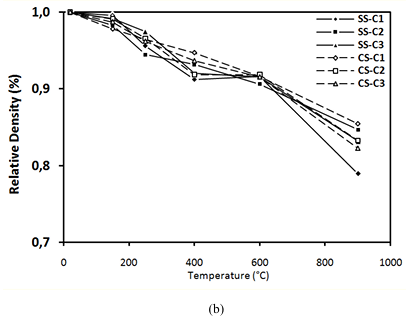
It is clear that the decrease of concretes containing SS is similar to that of concretes containing CS, about 8% at 600°C and between 16% and 17% at 900°C.
4.6. Volume
The analysis of the different volume variation curves presented in Figure 13 shows that the variation in concrete volume is influenced by the nature of sand.
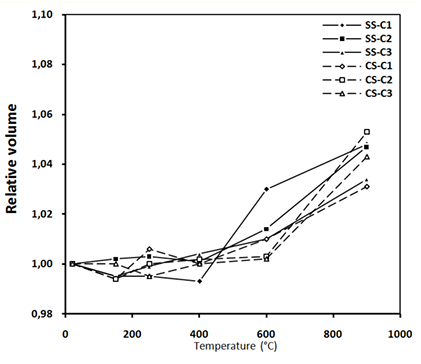
In the temperature range of 20 to 250°C, all concretes show a decrease in volumes, which results in the removal of water contains in the cement paste. At the beginning of the heating cycle, the cement paste expands and then shrinks; hence the shrinkage phase begins at a lower temperature (125°C) [39].
In the range of 400 to 900°C, the concretes containing SS have higher volume variations than concretes containing CS. This variation is related to the nature of the SS, which undergoes a volume increase of about 1% during the quartz change at 575°C, which contributes to the significant cracking of concrete [39].
4.7. Cracks
The evolution of cracks for different concretes after their cooling was followed by Hachemi [15]. The opening of cracks was determined using an optical microscope (Table 3).
Table 3. Widths of macro-cracks of different concretes after heating to 400°C, 600°C and 900°C [15].
Mixtures | Width of cracks (mm) | |||||
400°C | 600°C | 900°C | ||||
min | max | min | max | min | max | |
SS-C1 | < 0,05 | 0,08 | < 0,05 | 0,25 | < 0,05 | 0,70 |
SS-C2 | < 0,05 | 0,05 | < 0,05 | 0,10 | < 0,05 | 0,50 |
SS-C3 | < 0,05 | 0,05 | < 0,05 | 0,08 | < 0,05 | 0,35 |
CS-C1 | < 0,05 | 0,05 | < 0,05 | 0,10 | < 0,05 | 0,60 |
CS-C2 | < 0,05 | 0,05 | < 0,05 | 0,10 | < 0,05 | 0,50 |
CS-C3 | < 0,05 | 0,05 | < 0,05 | 0,10 | < 0,05 | – |
Figure 14 shows different cracks occurred in cement paste using the optical microscope.
Before 400°C, no cracks have been see with the naked eye on the specimens, but cracks of small aperture were observed (less than 0.05 mm). This damage can be caused by the high thermal expansion of water within the pores, which can induce high tensile stresses in the solid skeleton and possible cracks [40]. Then, concrete cracking develops progressively with increasing temperature.
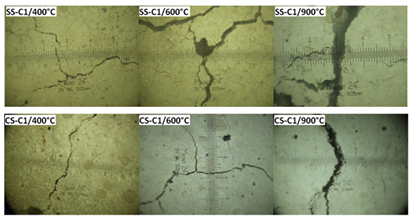
Concretes specimens heated to 400°C are characterized by the presence of open cracks. The crack opening varies from 0.04 to 0.08 mm depending on the type of concrete and sand used. Beyond this temperature, the amount and the opening of cracks become larger and visible to the naked eye. These cracks are located on the specimen’s surface and extend into the cement paste towards the inside of specimens. Concrete cracking, which manifests from the temperature of 400°C, is mainly due to the difference in the directions of the thermal deformation of cement paste (shrinkage) and aggregates (expansion) [39]. The opposite evolution of cement paste and aggregates generates deformative incompatibilities at the cement-aggregate interface, which generate tensile stresses in cement paste and compressive stresses in aggregates. This opposite behavior of aggregates-cement paste could therefore causes microcracks in the material [41].
After heating to 600°C, numerous network-connected cracks were observed; these cracks appear in the cement paste. The opening of these cracks was about 0.05 to 0.25 mm for concrete made with CS. Concrete specimens containing SS have the lowest crack openings.
After heating cycle of 900°C, the opening and the depth of the cracks become larger. Greater cracking is observed with an opening up to 0.70 mm. These crack openings could indeed be accompanied by loss of material which would affect the total mass loss of the specimens treated at this temperature.
Concrete specimens containing SS have the lowest crack openings. It is also observed that the opening of the cracks was smaller for concretes with lower w/c ratio.
5. Conclusion
The use of two different nature of sand in the manufacturing of concrete leads to a different behavior when exposed to high temperature.
For temperature higher than 600°C, concretes made with CS present greater mass loss than concretes made with SS, which can be explained by the decomposition of calcite CaCO3 and the departure of CO2. The carbonates of CS lead to an additional damage of concrete (higher porosity) when compared to concretes containing SS in the range temperature of 600 to 900°C.
The difference in the high temperature behavior of SS and CS leads to different thermal behavior of concrete. Concretes containing SS show an increase of volume higher than that of concrete containing CS after a heating to 600°C. This can be explained by the increase of SS volume due to transformation of quartz α to quartz β which occurs at 573°C. However, density of concretes does not depend on the nature of sand. The evolution of density of concretes made with SS was very close to that of concretes made with CS.
The evolution of residual compressive strength of concretes depends on the mineralogical nature of sand and the w/c ratio from 20° to 600°C. For a high w/c, CS-concretes have the best residual compressive strength while with a lower w/c; SS-concretes behave the best. For the UPV tests, concretes contained SS present the lowest values of UPV than that of concretes contained CS. In addition, CS-Concretes show less cracking than the SS-concretes.
Conflict of Interest
The authors declare no conflict of interest.
- C. De Sa, “Etude hydro-mécanique et thermo-mécanique du béton – Influence des gradients et des incompatibilités de déformation,” (Ph. D Thesis, Ecole Normale Supérieure de CACHAN, 2007).
- M. Bederina, Z. Makhloufi, A. Bounoua, T. Bouziani, M. Quéneudec, “Effect of partial and total replacement of siliceous river sand with limestone crushed sand on the durability of mortars exposed to chemical solutions,” Construction and Building Materials, vol. 47, pp. 146-158, 2013, doiI: 10.1016/j.conbuildmat.2013.05.037.
- T. Celik, K. Marar, “Effects of crushed stone dust on some properties of concrete,” Cement and Concrete Research, vol. 26, pp. 1121-1130, 1996, doi: 10.1016/0008-8846(96)00078-6.
- M. Yajurved Reddy, D. V. Swetha, S. K. Dhani, “Study on properties of concrete with manufactured sand as replacement to natural sand,” International Journal of Civil Engineering and Technology, vol. 6, pp. 29-42, 2015,
- N. Tebbal, Z. Rahmouni, “Influence of local sand on the physic-mechanical comportment and durability of high performance concrete,” Advances in Civil Engineering, vol. 8, pp. 1-10, 2016, doi: 10.1155/2016/3897064.
- T.T.H. Le, H. Boussa, F. Meftah, “Effect of aggregates morphology on the THM behaviour of concrete at high temperatures,” In: Proceedings of Fracture Mechanics of Concrete and Concrete Structures – High Performance, Fiber Reinforced Concrete, Special Loadings and Structural Applications (FraMCoS-7), pp. 1758-1765, 2010.
- R. Felicetti, P.G. Gambarova, “Expertise and assessment of materials and structures after fire,” Int : fib bulletin 46: fire design of concrete structures-structural behaviour and assessment. International Federation for Structural Concrete (fib) 1rst ed, pp. 63-114, 2008, doi : doi.org/10.35789/fib.BULL.0046.
- I. Hager, T. Tracz, J. Śliwiński, K. Krzemień, “The influence of aggregate type on the physical and mechanical properties of high-performance concrete subjected to high temperature,” Fire and Materials, vol.40, no. 5, pp. 668-682, 2015, doi:10.1002/fam.2318.
- J-C. Mindeguia, P. Pimienta, H. Carré, C. La Borderie, “On the influence of aggregate nature on concrete behavior at high temperature,” European Journal of Environmental and Civil Engineering, vol. 16, pp. 236-253, 2012, doi:10.1080/19648189.2012.667682.
- Z. Xing, R. Hébert, A-L. Beaucour, B. Ledésert, A. Noumowé, “Influence of chemical and mineralogical composition of concrete aggregates on their behaviour at elevated temperature,” Materials and Structures RILEM, vol. 47, pp. 1921-1940, 2014, doi: 10.1617/s11527-013-0161-y
- Z. P. Bazant, M. F. Kaplan, Concrete at High temperatures, Material properties and mathematical models, Concrete Design & Construction Series, 426 p. Longman Group Limited, 1996.
- G.A. Khoury, Y. Anderberg, K. Both, J. Fellinger, N.P. Hoj, C. Majorana, “Fire design of concrete structures-materials, structures and modeling,” State of art report, FIB Bulletin, N° 38, 2007, doi: 10.35789/fib.BULL.0038.
- J. Lee, K. Choi, K. Hong, “The effect of high temperature on color and residual compressive strength of concrete,” In: Proceedings of Fracture Mechanics of Concrete and Concrete Structures – High Performance, Fiber Reinforced Concrete, Special Loadings and Structural Applications (FraMCoS-7), pp. 1772-1775, 2010.
- AFNOR French standardization P 18-554, Aggregates – Measurement of densities, porosity, absorption coefficient and water content of fine gravel and pebbles, French Association for Standardization (AFNOR), Tour Europe cedex 7 92049, Paris, 1990.
- S. Hachemi, A. Ounis, “The influence of sand nature on the residual physical and mechanical properties of concrete after exposure to elevated temperature,” European Journal of Environmental and Civil Engineering, vol. 23, pp. 1003-1018, 2019, doi: 10.1080/19648189.2017.1327893.
- ISO/TR 15655, Fire resistance – Tests for thermo-physical and mechanical properties of structural materials at elevated temperatures for fire engineering design, Technical report, Geneva, 2003.
- European Standard NF EN 12390-7, Test for hardened concrete Part 7: Density of concrete, ISSN 0335-3931, The French Association of Standardization (AFNOR), 11 avenue Francis de Pressensé 93571 Saint-Denis La Plaine Cedex, 2001.
- European Standard NF EN 12390-3, Test for hardened concrete Part 3: Compressive strength of test specimens, ISSN 0335-3931, The French Association of Standardization (AFNOR), 11 avenue Francis de Pressensé France 93571 Saint-Denis La Plaine Cedex, 2003.
- French Association for Standardization AFNOR P 18-418, Concrete – Sonic auscultation – measurement of the sonicwave transmission time in concrete, Tour Europe cedex 7 92080, Paris defense, 1989.
- B. A. Scherefler, D. Gawin, G. A. Khoury, C. E.Majorana, “Physical, Mathematical & numerical modeling,” Course on Effect of Heat on Concrete. International Centre for Mechanical Sciences (CISM), 2003.
- C. Alonso, C. Andrade, M. Castellote, G. A. Khoury, “Microstructure – Solid Phases,” Course on Effect of Heat on Concrete. International Centre for Mechanical Sciences (CISM), 2003.
- V. Wetzig, “Destruction mechanisms in concrete material in case of fire, and protection systems,” In: 4th Int. Conf. on Safety in Road & Rail Tunnels (SIRRT), pp. 281-290, 2001.
- J. P. Ingham, “Application of petrographic examination techniques to the assessment of fire-damaged concrete and masonry structures,” Materials Characterization, vol. 60, pp. 700-709, 2009, doi: 10.1016/j.matchar.2008.11.003.
- S. Hachemi, “Etude du comportement du béton soumis à haute température : Influence du type de béton et de la nature des constituants,” (Ph. D Thesis, Université de Biskra, 2015).
- S. Hachemi, A. Ounis, “L’influence de la nature du sable sur les propriétés physiques et mécaniques du béton soumis à haute température,” Courrier du Savoir, Université de Biskra, Algérie, vol. 24, pp. 151-162, 2017.
- Z. Xing, A-L. Beaucour, R. Hebert, A. Noumowe, B. Ledesert, “Influence of the nature of aggregates on the behaviour of concrete subjected to elevated temperature,” Cement and Concrete Research, vol. 41, pp. 392-402,2011, doi:10.1016/j.cemconres.2011.01.005.
- R. Niry, A-L. Beaucour, R. Hebert, A. Noumowe, B. Ledesert, R. Bodet, “Thermal stability of different siliceous and calcareous aggregates subjected to high temperature,” MATEC Web of Conferences,vol. 6, pp. 1-9, 2013, doi:10.1051/matecconf/20130607001.
- Z. Xing, R. Hébert, A-L. Beaucour, B. Ledésert, A.Noumowe, “Influence of chemical and mineralogical composition of concrete aggregates on their behaviour at elevated temperature,” Materials and Structures RILEM,vol. 47, pp. 1921-1940, 2013, doi : 10.1617/s11527-013-0161-y.
- M. Khattab, S. Hachemi, M.F. Al Ajlouni, “Evaluating the physical and mechanical properties of concrete prepared with recycled refractory brick aggregates after elevated temperatures’ exposure, ” Construction and Building Materials, 311, 2021, https://doi.org/10.1016/j.conbuildmat.2021.125351
- Tsymbrovska, “Effect of heating–cooling cycles on transient creep strain of high performance,” high strength and ordinary concrete under service and accidental conditions materials and structures,vol. 48, pp. 1561-1579, 1998, doi : 10.1617/s11527-014-0254-2.
- N. Tebbal, Z. RAHMOUNI, M. Maza, “Combined effect of silica fume and additive on the behavior of high performance concretes subjected to high temperatures,” mining science, vol. 24, pp. 129-145, 2017, doi : 10.5277/msc172408.
- I. Hager, “Behaviour of cement concrete at high temperature,” Bulletin of the Polish Academy of Sciences: Technical Sciences, vol. 61, pp. 145-154, 2013, doi:10.2478/bpasts-2013-0013.
- S. Rao, K. Rahul, A. Pradesh, “Studies on bacterial concrete exposed to elevated temperatures and thermal cycles,” Studies, vol. 3, pp. 126-135, 2013.
- M. Belouadah, Z. Rahmouni, N. Tebbal, “Effects of glass powder on the characteristics of concrete subjected to high temperatures,” Advances in Concrete Construction, vol. 6, pp. 311:322, 2018, doi: 10.12989/acc.2018.6.3.311
- G. Verbeck, L.E. Copeland, “Some physical and chemical aspects of high pressure steam curing,” In: Menzel Symposium on High Pressure Steam Curing (ACI SP-32), pp. 1–131, 1972, doi:10.14359/6597.
- Z. Rahmouni, N. Tebbal, H. Haroun Abdellah, “Influence de la nature des granulats sur le comportement rhéologique du béton à hautes températures, ” MATEC Web of Conferences, vol. 01, pp. 04-11, 2014, doi: 10.1051/matecconf/20141101010.
- M. Khattab, S. Hachemi, H. Benzetta, “Assessment of quality of recycled brick concrete using Ultrasonic pulse velocity, ” ASPS Conference Proceedings, First International Conference on Energy, Thermofluids and Materials Engineering, ICETME 2021.
- Z. Xing, “Influence de la nature minéralogique des granulats sur leur comportement et celui du béton à haute température,” (Ph. D Thesis, Université de Cergy-Pontoise, 2011).
- G. I. Hager, “Comportement à haute température des bétons à haute performance – Evolution des principales propriétés mécaniques,” (Ph. D Thesis, Ecole Nationale des Ponts et Chaussées et l’Ecole Polytechnique de Cracovie, 2004).
- J-C. Mindeguia, “Contribution expérimentale a la compréhension des risques d’instabilité thermique des bétons,” (Ph. D Thesis, Université de Pau et des Pays de l’Adour, 2009).
- P. Pliya, “Contribution des fibres polypropylène et métalliques à l’amélioration du comportement du béton soumis à une température élevée,” (Ph. D Thesis, Université de Cergy-Pontoise, 2010).
- Samya Hachemi, Zine Elabidine Rahmouni, “Education and Sustainability Habits – Portuguese Students’ Perspectives”, Journal of Engineering Research and Sciences, vol. 4, no. 7, pp. 15–25, 2025. doi: 10.55708/js0407002
- Samya Hachemi, Zine Elabidine Rahmouni, “DC/DC Converter by using FPGA”, Journal of Engineering Research and Sciences, vol. 3, no. 3, pp. 13–18, 2024. doi: 10.55708/js0303002
- Samya Hachemi, Zine Elabidine Rahmouni, “Using Interaction Geography to Explore Building Occupant Behaviors in Virtual Reality: A Pilot Study”, Journal of Engineering Research and Sciences, vol. 1, no. 11, pp. 1–7, 2022. doi: 10.55708/js0111001
- Samya Hachemi, Zine Elabidine Rahmouni, “Competency Manifestation Clues within Interactions in Computer Mediated Communication”, Journal of Engineering Research and Sciences, vol. 1, no. 5, pp. 167–178, 2022. doi: 10.55708/js0105018
