Failure Analysis & Mechanism Studies of the Worm-like Defects in Vias of Wafer Fabrication
Journal of Engineering Research and Sciences, Volume 2, Issue 12, Page # 1-6, 2023; DOI: 10.55708/js0212001
Keywords: Failure analysis, Cl corrosion, The Worm-like defects, Vias and wafer fabrication
(This article belongs to the Section Electrical Engineering (ELE))
Export Citations
Cite
Younan, H. , Lois, L. J. , Binghai, L. , Lei, Z. and Xiaomin, L. (2023). Failure Analysis & Mechanism Studies of the Worm-like Defects in Vias of Wafer Fabrication. Journal of Engineering Research and Sciences, 2(12), 1–6. https://doi.org/10.55708/js0212001
Hua Younan, Liao Jinzhi Lois, Liu Binghai, Zhu Lei and Li Xiaomin. "Failure Analysis & Mechanism Studies of the Worm-like Defects in Vias of Wafer Fabrication." Journal of Engineering Research and Sciences 2, no. 12 (December 2023): 1–6. https://doi.org/10.55708/js0212001
H. Younan, L.J. Lois, L. Binghai, Z. Lei and L. Xiaomin, "Failure Analysis & Mechanism Studies of the Worm-like Defects in Vias of Wafer Fabrication," Journal of Engineering Research and Sciences, vol. 2, no. 12, pp. 1–6, Dec. 2023, doi: 10.55708/js0212001.
In semiconductor wafer fabrication, the contamination issue by halogens (such as F, Cl, and Br) brings great challenges to metallization processes in the back end of line (BEOL). For aluminum (Al) back-end process, severe metal corrosion may occur by halogens, forming aluminum halide defects. Such halogen-induced metal corrosion issue creates the defects on Al metal lines, Al bondpads and Vias, causing direct device failure, resulting in low yield and reliability issue. In this paper, we reported failure analysis and mechanism studies of the Worm-like defects in Via holes, which was caused by Cl contamination and the subsequent Al metal corrosion. With this study, a corrosion mechanism was proposed in which a Cl-based chemical chain-reaction resulted in repeated/continuously enhanced chemical corrosion even with a small amount of Cl ions. The chemical chain-reactions caused more serious corrosion with corrosive by-products. Such Cl-induced Al metal corrosion and the worm-like defect formation resulted in the process integrity issues related to Al metallization processes, such as voiding in Via structures, opening or bridging in between metal lines.
1. Introduction
In semiconductor wafer fabrication, the contamination issue by halogens (such as F, Cl, and Br) brings great challenges to metallization processes in the back end of line (BEOL). Table 1 indicates the typical plasma etch gases used for each type of material in wafer fabrication (fab), which may cause Cl, F and Br cross contamination [1].
Table 1: Typical plasma etch gases for polysilicon, SiO2 and Al films
Material | Etchant |
Polysilicon | CF4 CF4/H2 CF4/O2, SF6 HBr, Cl2, Cl2/HBr/O2 |
SiO2 | SF6, NF3, CF4/O2, CF4 |
Al | Cl2 Cl2/CHCl3, Cl2/N2 |
For aluminum (Al) back-end process, severe metal corrosion may occur by halogens, forming aluminum halide defects. Such halogen-induced metal corrosion issue creates the defect on Al metal lines, Al bondpads and Vias, causing direct device failure, resulting in low yield and reliability issue.
In our previous papers, we have performed failure analysis and mechanism studies on fluorine (F) contamination and their Al halide defects. We have presented detailed studies on the failure mechanisms and failure models, and proposed corrective and preventive measures, as well as process monitoring solutions which are useful in semiconductor industries and wafer fab [2-5].
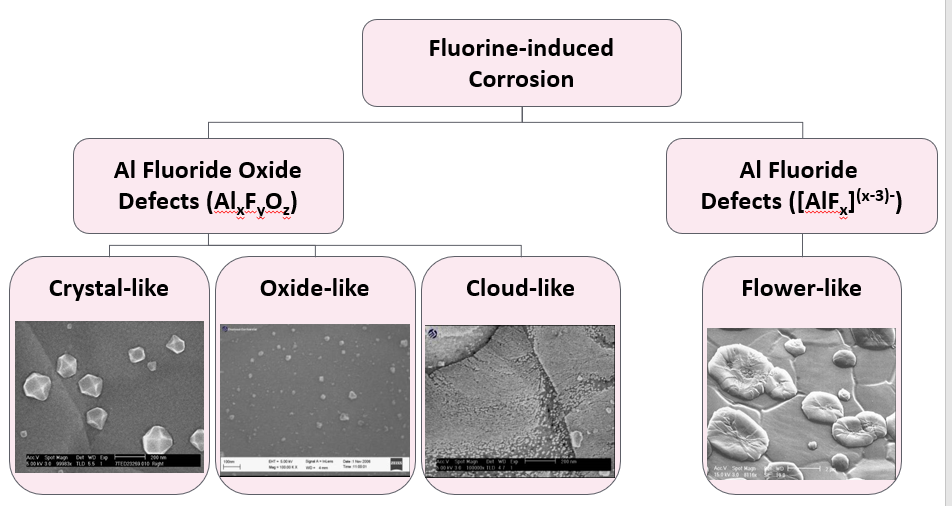
For example, we have deeply studied the F-induced corrosion defects and proposed the related failure models, and made classification for the F-induced corrosion defects on the Al bondpads as the “flower-like”, “crystal-like”, “oxide-like” and “cloud-like” defects as shown in Figure 1, which have been characterized using SEM/EDX, TEM, Auger, XPS and TOF-SIMS analysis techniques [4].
In this paper, we will perform failure analysis and mechanism studies of the Cl-induced defects, especially for the Worm-like defects in Via holes after Via etching process in wafer fab, which is caused by chlorine (Cl) contamination and corrosion in wafer fab during Via processes.
2. Cl-Induced Corrosion and Failure Analysis of the Worm-Like Defects in Via Holes
In wafer fab, the control of Cl contamination in production should be very strict as it will result in severe metal Cl corrosion. Cl contamination and the formation of corrosion defects is prone to occur in Al metallization and Vias forming processes, because Cl-containing gases (such as Cl2, CHCl3, BCl3, etc.) are used in Al metallization BEOL processes in wafer fab. After fab processes, certain amount of Cl may not be completely cleaned away, leaving behind the corrosive Cl-containing residue or contaminants although the cleaning processes are employed after metal etching process. Moreover, sometime, Cl contamination may be from wafer fab environment or/and from the mini-environment of SMIF pods, which is used to transport.
2.1. Cl Contamination and Corrosion
Once Cl contamination occurs in wafer fab, Cl will chemically react with Al to form Al chloride. Its typical chemical reaction formula is:
$$\text{Al} + 3\text{Cl}^- \rightarrow \text{AlCl}_3 + 3e^- \tag{1}$$
$$\text{(Water soluble)}$$
Based on our study results and the associated Cl-based chemistry, the chemical reactions and corrosion between Cl and Al metal are very fast and intensive ones, unlike those chemical corrosion induced by F contaminant in which the initiation of F-based corrosion may need certain period of time, e.g., hours usually.
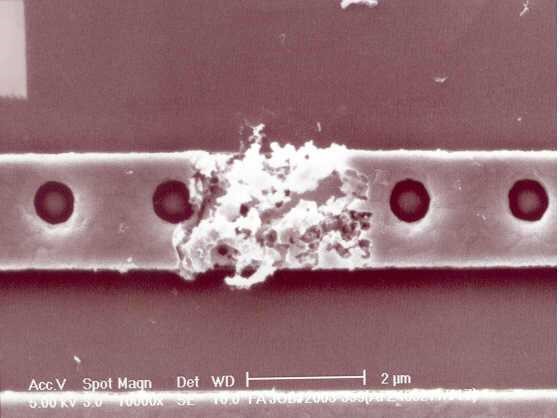
Figure 2 shows a Cl corrosive defect on the Al metal line. One can see that the defect has damaged the metal line, which may cause low yield or reliability issue. The root cause was identified to be Cl environment contamination. It is understood that differencing from F-induced corrosion, Cl-induced corrosion is immediate. Moreover, AlCl3 is a chemically unstable compound and it dissolves in water. Therefore, Cl-induced corrosion is more serious as it can cause chemical chain reactions (see discussion in the next session).
2.2. The Worm-like Defects in Al Via Holes
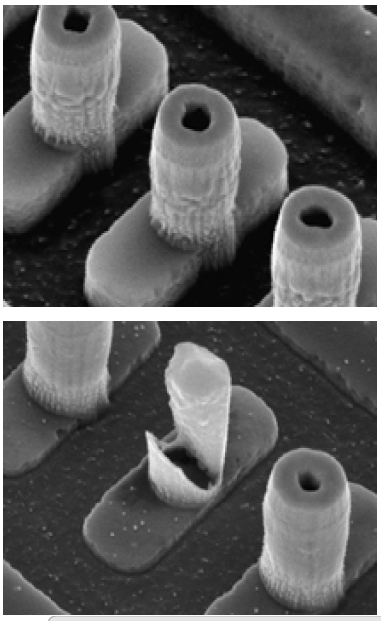
As all we know, in typical Al-based BEOL processes, W Vias structures serve as the interconnects in between different Al metal layers, as shown in Figure 3. The formation of W vias involved somewhat complicated process sequences, from the initial Al metal line patterning, to IMD CVD processes, to via hole etch and etch, barrier-layer deposition and W filling, and to the final W CMP processes. Any issue related to the integrity of the above-mentioned processes may lead to the direct device failure, causing low yield and reliability failure.
In this case, we reported an interesting case study related to Cl contamination and the associated Al metal corrosion, i.e., the worm-like defect formation after Via hole etching process in wafer fab.
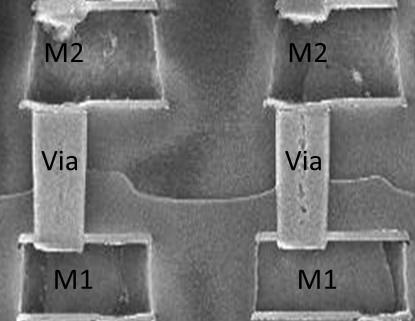
In this case, we reported an interesting case study related to Cl contamination and the associated Al metal corrosion, i.e., the worm-like defect formation after Via hole etching process in wafer fab.
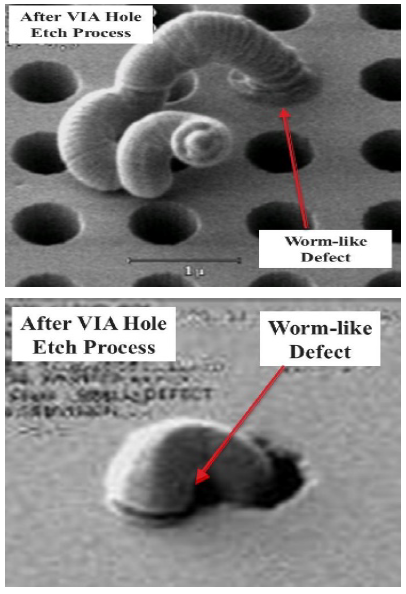
Inline SEM inspection after VIA hole etch process revealed the formation of bountiful worm-like defects, protruding from via holes, as shown by the SEM images in Figure 4. With the presence of these worm-like defects will definitely affect the subsequent processes like deposition of the barrier layers and W layer. In order to the understand the root-cause behind the formation worm-like defects, we performed detailed failure analysis and failure mechanism studies.
3. Failure analysis and mechanism studies of the worm-like defects in via holes
Figure 5 shows the micro-area EDX analysis on the worm-like defect. As shown in Figure 5, a small amount of Cl was detected, indicating that the worm-like defect was formed due to Cl-induced corrosion. Further FIB-SEM analysis showed that the worm-like defects directly grew from the bottom Al M1 layer along the via holes.
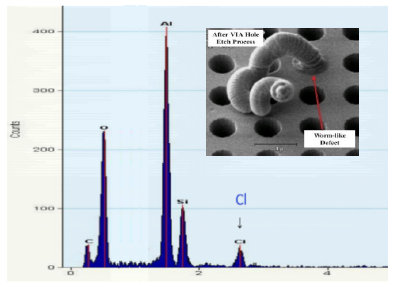
Nonetheless, it was still difficult to understand how these Cl-corrosion worm-like defects were so intensive that the corrosion-induced by products were able to protrude from the bottom M1 to top via hole surface with long tails. Here, in term of Cl-based chemistry, we proposed the Cl chain chemical reactions theory and these worm-like defects were not due to simple chemical corrosion reaction, but due to the Cl chain chemical reactions, where only a small amount of Cl ions could propagate in chemical reactions repeatedly, and finally it causes very intensive Cl corrosion reactions even with a small amount of Cl ions.
3.1. Failure Mechanism
After performing studies, we have understood that:
- a) Cl-corrosion is self-sustained;
- b) A small amount of Cl ions is sufficient to trigger the
corrosion; - c) Cl and Al form Al chloride (AlCl3) which is water-
Cl contamination chemically reacts with Al to form AlCl3 as Eqn (1), which is a chemically unstable compound and it is water soluble. Therefore, AlCl3 can further chemically react with water or moisture (H2O) and form hydrated Aluminium hydroxide product (Al(OH)3) and further release Cl– ions:
$$\text{AlCl}_3 + 3\text{H}_2\text{O} \rightarrow \text{Al(OH)}_3 + 3\text{Cl}^- + 3\text{H}^+ \tag{2}$$
The new product of hydrated Aluminium hydroxide product (Al(OH)3) will be discomposed and forms Al oxide (Al2O3):
$$\text{Al(OH)}_3 \rightarrow \tfrac{1}{2} \text{H}_2\text{O} + \tfrac{1}{2} \text{Al}_2\text{O}_3 \tag{3}$$
Because AlCl3 is water-soluble, it can release Cl ions again when it encounters water vapor, and further corrodes Al metal. Therefore, a small amount of Cl ions could repeatedly propagate in chemical reactions, causing a Cl chain chemical reaction, which produces alumina, a corrosion product:
$$2\text{Al} + 6\text{Cl}^- \rightarrow 2\text{AlCl}_3 \rightarrow 2\text{Al(OH)}_3 + 6\text{Cl}^- + 6\text{H}^+ \xrightarrow{-3\text{H}_2\text{O}} \text{Al}_2\text{O}_3 \tag{4}$$
$$n\text{Al}_2\text{O}_3 + m\text{AlCl}_3 \rightarrow \text{Al}_x\text{Cl}_y\text{O}_z \quad \text{(Worm-like defects)} \tag{5}$$
In the production process of wafer fab and via holes, Al of M1 reacts with Cl to generate aluminium trichloride (AlCl3). Because aluminium trichloride is water soluble. Therefore, when it encounters water vapor, a further chemical reaction can produce HCl, which is later decomposed into Cl ions again. The Cl ions could further react with Al to form aluminium trichloride. This repeated chemical reaction process is so-called “Cl-chain chemical reaction”. As a result of the “Cl-chain chemical reaction”, only a small amount of Cl ions could propagate in chemical reactions repeatedly, and finally it causes very intensive Cl corrosion reactions even with a small amount of Cl ions.
On the other hand, during chemical reactions, one of the by-products from the chemical reaction is aluminium hydroxide. In the production process, aluminium hydroxide would be dehydrated to form alumina. These aluminium oxides and part of aluminium trichloride will generate aluminium oxychloride (AlxClyOz) through a series of physical and chemical reactions.
With the progress of the chemical reactions, the mixtures of alumina and aluminium oxychloride would continuously grow up, just like worms slowly formed and crawled out of via holes, forming the worm-like defects as observed. Finally, the reaction in between Alumina and Al chloride produced aluminium oxychloride (AlxClyOz). They could grow up from the bottom of the Vias, and eventually form the worm-like defects in and outside the Via holes.
3.2. Failure Analysis
In via process of wafer fabrication, the worm-like defects could occupy the entire via holes or part of the via holes. In the subsequent via metal filling, some vias would show partial via filling, resulting in empty via or via hole issues, which in turn directly the BOEL process integrity.
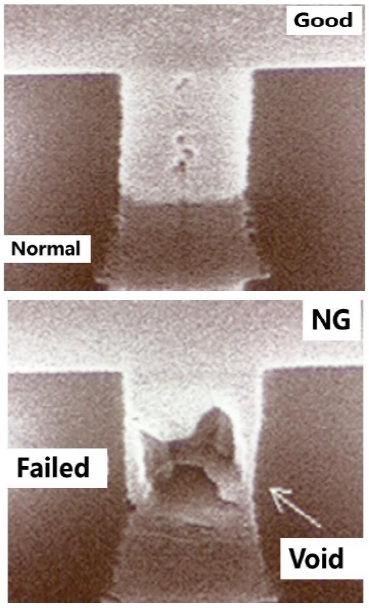
For the failure via structures, we performed the failure analysis by deprocessing (delayering), and then top-down and cross-sectional SEM analysis. Figure 6 showed the top-down SEM images after deprocessing. As shown by Figure 6, the good Vias essentially showed good metal filling (up of the Figure 6), but in the bad area, one Via showed apparent partial filling due to the worm-like defect (down of Figure 6).
We also performed cross-section FIB (focused ion beam)-SEM analysis. The cross-sectional SEM analysis results were shown in Figure 7. It is clear that the via in the affected lots showed partial filling with a big void at the via bottom (down of the Figure 7) and the FIB-SEM result showed that in the good sample without the worm-like defect, the Via was completed to fill in (up of the Figure 7).
3.3. C. Root Cause Identification and Solution
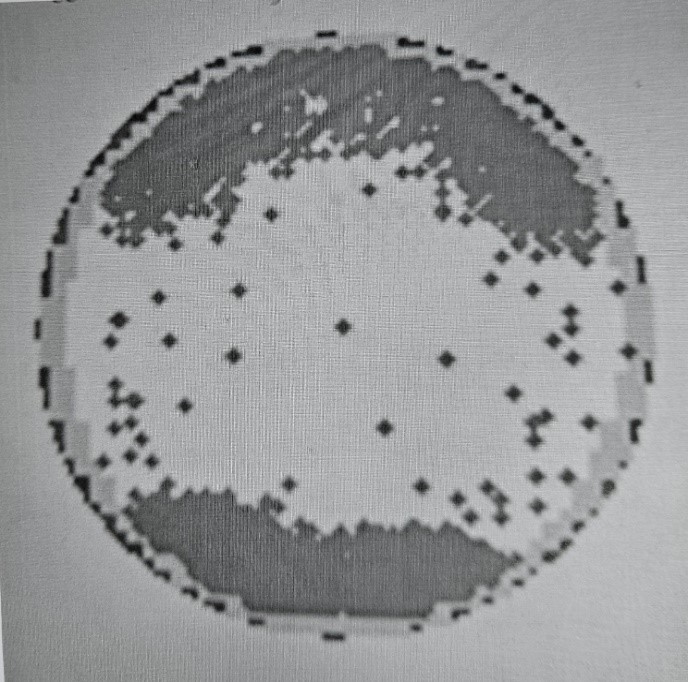
Inline investigations and analysis reveal interesting characteristics of the worm-like defects. KLA scan maps of the affected wafers showed a distinct bracket signature (refer to Figure 8). There was also no single wafer orientation or alignment with respect to the notch. Another characteristic was that the first slot was typically the most severely affected wafer compared to the rest of the wafers in the lot. It has also been observed that the occurrence of the worm-like defects was intermittent with no common tool, device/part, or layer. It also occurred even with environmental Cl levels safely below the specification limit.
Based on the abovementioned characteristics of the defect, residual chlorine is believed to have originated from the mini-environments (SMIF pod) used to transport the wafers during IC fabrication. After etch, the photoresist is removed by plasma resist strip (PRS) process. In the PRS tool, there is a possibility of pod switching. For instance, a SMIF pod may contain wafers that have undergone polysilicon etch which uses Cl-based etchant gases (refer to Table 1). As a result, the SMIF pod traps residual Cl. After the PRS step, it is possible for wafers with exposed Al metal to be contained in a Cl-contaminated SMIF pod that was previously used during polysilicon etch, a Cl-based etch process. The residual Cl inside the contaminated pod attacks the exposed Al metal in the presence of ambient moisture and forms corrosion products as discussed in the earlier section.
The pod-switching scenario described above occurs at random. This explains why only a few and very intermittent cases occur, and that no common tool, device/part or layer has been derived. Furthermore, worm-like defects are only seen after via etch and not after metal etch. This is because metal etchers employ in situ resist strip where pod switching is not possible.
The distinct bracket signature in KLA scan maps matches the exposed sections of the wafers when placed inside the cassette (see Figure 4). In other words, only those sections exposed to the SMIF pod are affected with worm-like defects.
As the topmost wafer is the most exposed wafer, it is also the most severely affected, hence producing the “first wafer” effect. These observations reinforce the theory that corrosion is due to SMIF pod contamination. A feasible solution therefore is to eliminate the possibility of pod switching between a pod used for Cl-based etch process and a pod with exposed Al metal.
4. Conclusions
In this work, we performed failure analysis and mechanism studies of the Worm-like defects in Via holes of wafer fab. The Worm-like defects occurred after Via holes etching processes, which was caused by Cl contamination and corrosion. We proposed Cl-chain chemical reaction mechanism related to Cl-induced chemical corrosion processes: a). Cl-corrosion is self-sustained; b). A small amount of Cl is sufficient to trigger corrosion; and c). Cl and Al form Al chloride (AlCl3). Because AlCl3 is water-soluble, it can release Cl ions again when it encounters water vapor, then further corroding Al metal. Therefore, a small amount of Cl ions could be repeatedly used in chemical reactions, causing a Cl-chain chemical reaction, which produces alumina, a corrosion product. These aluminum oxides and part of aluminum trichloride would generate aluminum oxychloride (AlxClyOz) through a series of physical and chemical reactions. These by-products induced by Cl-chain reaction processes accumulated and grew from the bottom of the Vias, and eventually formed the Worm-like defects in and outside the Via holes. In the subsequent via metal filling, some vias would appear that the metal filling would not enter that cause voids in Vias and finally after M2 went up, it would cause M1 and M2 to open or leak directly.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgment
The authors would like to thanks FA personnel from Wintech-Nano for their technical support.
- C. J Mogab, “Dry Etching,” in VLSI Technology, ed by S.M. Sze, McGraw-Hill, pp. 307 (1983).
- H. Younan et. al., “A Study on Non-Stick Aluminum Bondpads due to Fluorine Contamination using SEM, EDX, TEM, IC, Auger, XPS & TOF-SIMS Techniques”. The proceedings from the 28th International Symposium for Testing & Failure Analysis (ISTFA’2002), 3-7 Nov, Phoenix Civic Plaza, Phoenix, Arizona, USA p495-504 (2002).
- H. Younan et. al., “A Study on Fluorine-Induced Corrosion on Microchip Aluminium Bondpads.” The proceedings from the 29th International Symposium for Testing & Failure Analysis (ISTFA’2003), 2-6 Nov. 2003, Santa Clara, California, USA, p249-255 (2003).
- H. Younan et. al., “Studies of Fluorine-induced Corrosion Defects on Microchip Al Bondpads and Elimination Solutions.” The Proceedings of the 34th International Symposium for Testing and Failure Analysis (ISTFA 2008), Nov 2-6, Portland, Oregon, USA, p285-290 (2008).
- H. Younan, “Studies and Application of Auger Monitoring System for Quality Control and Assurance of Al Bondpads.” The Proceedings of the 42nd International Symposium for Testing and Failure Analysis (ISTFA 2016), November 6-10, 2016, Fort Worth Convention Centre, Fort Worth, TX, USA (2016).
- Hua Younan, Liao Jinzhi Lois, Liu Binghai, Zhu Lei, Li Xiaomin, “A Study on the Bromine-induced Corrosion/Defects in Wafer Fabrication”, Journal of Engineering Research and Sciences, vol. 3, no. 2, pp. 8–14, 2024. doi: 10.55708/js0302002
